35 Worksheet 7 Oxidation Reduction Reactions Answers
Redox reaction worksheet. Subject: Chemistry. Age range: 14-16. Resource type: Worksheet/Activity. 4.7 3 reviews. ktbax263. 4.671428571428572 11 reviews. Last updated.. This is excellent, a really useful worksheet. Would love to have the answers too if possible next time! Thanks again! Show replies Combustion reactions chem worksheet 10 6 answers. Double Replacement Reactions For each of the following reactions: Predict the products, determine if it will react, and balance. Calculate the energy associated with this reaction. Many chemical reactions can be classified as one of five basic types.
Balancing Redox Equations Method 2: Half-reaction method 1. Divide the skeleton reaction into two half-reactions, each of which contains the oxidized and reduced forms of one of the species 2. Balance the atoms and charges in each half-reaction - Atoms are balanced in order: atoms other than O and H, then O, then H

Worksheet 7 oxidation reduction reactions answers
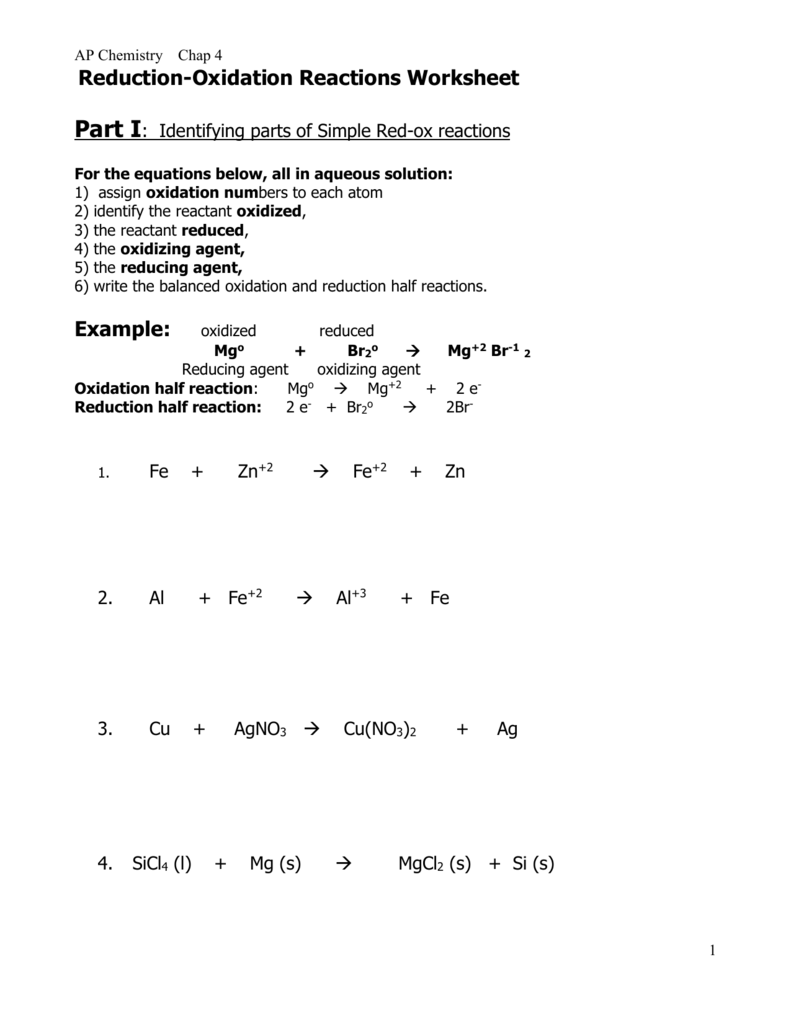
Balancing Redox Reactions Oxidation/Reduction (Redox) reactions can be balanced using the oxidation state changes, as seen in the previous example. However, there is an easier method, which involves breaking a redox reaction into two half- reactions. This is best shown by working an example. Oxidation-Reduction Worksheet. For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, and the reducing agent. 1) Mg + 2HCl ( MgCl2 + H2. 2) 2Fe + 3V2O3 ( Fe2O3 + 6VO.. Oxidation Reduction Worksheet Answers... Combustion reactions chem worksheet 10 6 answers. Combustion reactions chem worksheet 10 6 answers.
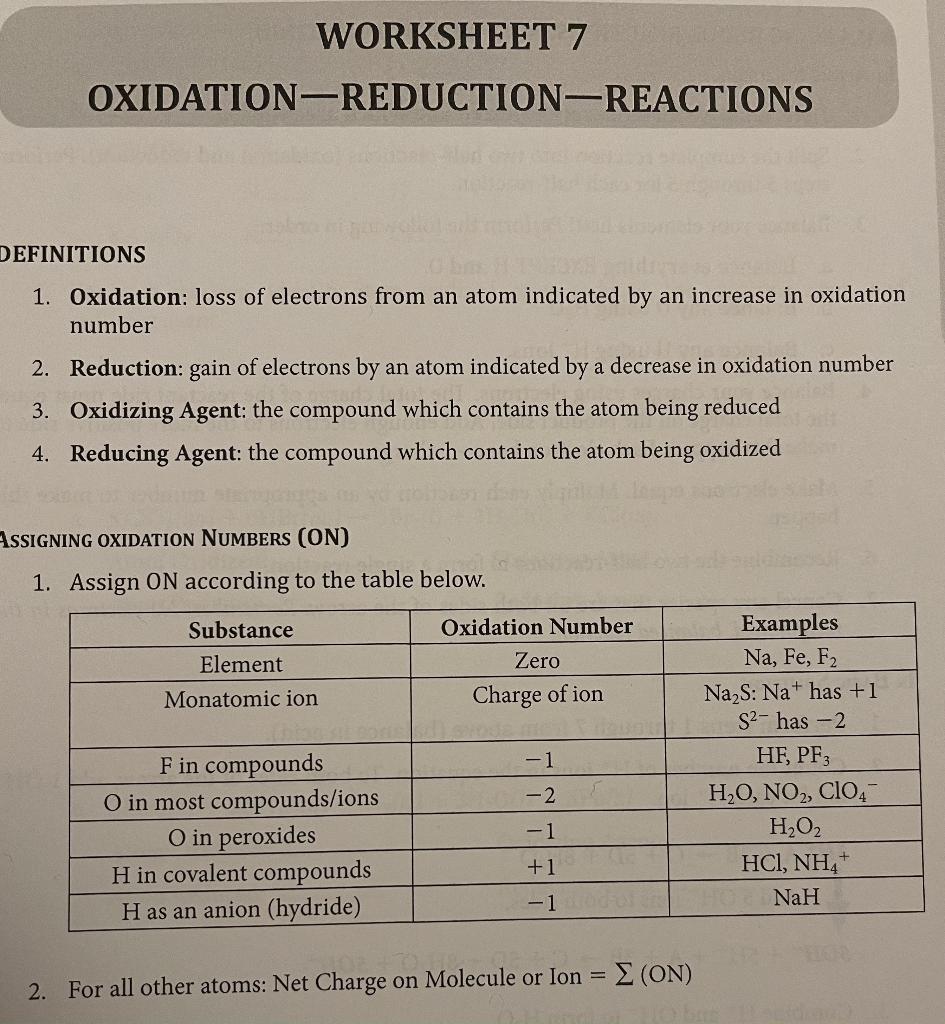
Worksheet 7 oxidation reduction reactions answers. Worksheet 7 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II – In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen –usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1.. This worksheet and quiz let you practice the following skills: Reading comprehension - ensure that you draw the most important information from oxidation and reduction reactions in the metabolism. Oxidation numbers are very important and are used for 1) naming compounds, 2) balancing oxidation-reduction reactions, 3) calculations in electrochemistry and other areas of chemistry. Rule 0 The following rules are in the form of a hierarchy; that is, the first stated rule takes What are the steps to balancing a redox reaction using the ½ reaction method? 1. Break the reaction up into two half reactions. One for is the reduction and the other is the reduction. 2. Balance all elements in the reaction except for oxygen and hydrogen. 3. Balance the oxygen by adding H 2 O. 4. Balance the hydrogen by adding H +. 5.
Write balanced equations for the following redox reactions: a. 2 NaBr + Cl 2 2 NaCl + Br 2 b. Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 in acidic solution c. 5 CO + I 2 O 5 5 CO 2 + I 2 in basic solution ; Write balanced equations for the following reactions: a. Cr(OH) 3 + Br 2 CrO 4 2-+ Br-in basic solution 10 OH-+ 2 Cr(OH) 3 + 3 Br 2 2 CrO 4 2-+ 8 H 2 O. Redox Half Reactions and Reactions WS #2 1. State the Oxidation Number of each of the elements that is underlined. a) NH 3-3 b) H 2SO 4 6 c) ZnSO 3 4 d) Al(OH) 3 3 e) Na 0 f) Cl 2 0 g) AgNO 3 5 h) ClO 4-7 i) SO 2 4 j) K 2Cr 2O 4 3 k) Ca(ClO 3) 2 5 l) K 2Cr 2O 7 6 m) HPO 3 2-3 n) HClO 1 o) MnO 2 4 p) KClO 3 5 q) PbO 2 4 r) PbSO 4 2 s) K 2SO 4 6. Oxidation-Reduction Worksheet. For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, the reducing agent, the oxidation half reaction, the reduction half reaction, and then balance the equation by the method of oxidation-reduction showing all electrons transfers. Sep 22, 2021 · Oxidation is a chemical reaction that occurs in an atom or compound and results in the loss of one or more electrons. Take a deeper look into the definition, process, and real-world examples of.
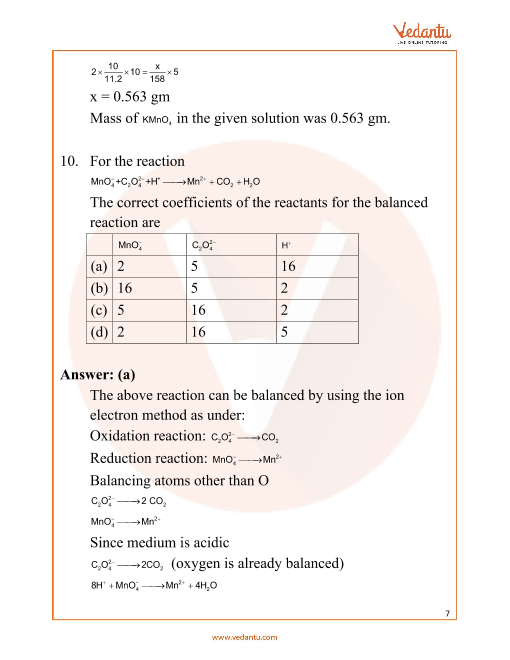
WORKSHEET 7 OXIDATION-REDUCTION-REACTIONS DEFINITIONS 1. Oxidation: loss of electrons from an atom indicated by an increase in oxidation number 2. Reduction: gain of electrons by an atom indicated by a decrease in oxidation number 3. Oxidizing Agent: the compound which contains the atom being reduced 4. Worksheet 25 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of 0 Group I and II – In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2. Hydrogen –usually +1, except when bonded to Group I or Group II, when it forms hydrides, -1.. 7 Oxidation, reduction, and redox reactions Answers to practice questions 4 (a) +2 +5 1 1 N has variable oxidation states, but oxygen is −2 only. 4 (b) NO3− + 4H+ + 3e− → NO + 2H 2 O 1 When the question says ‘in acid solution’ just put H+ ions in on the left and balance them up at the end. Recognizing redox reactions worksheet answers. 3mg n2 mg3n2 5. Balance each of the following half cell reactions. Suited for student in y10 and y11. In the reaction mg cl2 mgcl2 the correct half reaction for the oxidation that occurs is a. Redox reactions are a chemical reaction in which electrons are exchanged through oxidation and reduction.
Chapter 20 Worksheet: Redox I. Determine what is oxidized and what is reduced in each reaction. Identify the oxidizing agent and the reducing agent, also. 1. 2Sr + O2 2SrO 2. 2Li + S Li2S 3. 2Cs + Br2 2CsBr 4. 3Mg + N2 Mg3N2 5. 4Fe + 3O2 2Fe2O3 6. Cl2 + 2NaBr 2NaCl + Br2 7. Si + 2F2 SiF4 8. 2Ca + O2 2CaO 9.
Check 25+ pages worksheet 7 oxidation reduction reactions answers analysis in PDF format. SO 4 2-S 2- reducing agent c. Oxidation reduction worksheet for each reaction below identify the atom oxidized the atom reduced the oxidizing agent the reducing agent the oxidation half reaction the reduction half reaction and then balance the equation by the method of oxidation reduction showing all.
Oxidation-Reduction Worksheet. For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, and the reducing agent. 1) Mg + 2HCl ( MgCl2 + H2. 2) 2Fe + 3V2O3 ( Fe2O3 + 6VO.. Oxidation Reduction Worksheet Answers...
Access Free Chapter 20 Oxidation Reduction Reactions Worksheet Answers eBooks from the genres page or recommended category. Chapter 20 Oxidation Reduction Reactions Chapter 20: Oxidation-Reduction Reactions. The oxidation number of hydrogen in a compound is, except in metal hydrides, such as NaH, where it is -1. Chapter 20: Oxidation-Reduction.
Oxidation and Reduction Reactions Workbook Reference sheets: The activity series of common metals Calculating oxidation numbers Work sheets 1. Oxidation, Reduction, Agents, & Reactions. WS 1 2. Oxidation Numbers Spontaneous Reactions WS 2 3. Oxidation Numbers, Application to Reactions. WS 3
Balancing REDOX Reactions: Learn and Practice Reduction-Oxidation reactions (or REDOX reactions) occur when the chemical species involved in the reactions gain and lose electrons. Oxidation and reduction occur simultaneously in order to conserve charge. We can "see" these changes if we assign oxidation numbers to the reactants and products.
Combustion reactions chem worksheet 10 6 answers. Combustion reactions chem worksheet 10 6 answers.
Worksheet # 5 Balancing Redox Reactions in Acid and Basic Solution Balance each half reaction in basic solution. 4. Cr 2O 7 2 - → Cr3+ 5. NO → NO 3-6. SO 4 2- → SO 2 7. MnO 2 → Mn 2O 3 Balance each redox reaction in acid solution using the half reaction method. 8. H 2O 2 + Cr 2O 7 2- → O 2 + Cr 3+ 9. TeO 3 2-+ N 2O 4 → Te + NO 3-10.
Balancing Redox Reactions Oxidation/Reduction (Redox) reactions can be balanced using the oxidation state changes, as seen in the previous example. However, there is an easier method, which involves breaking a redox reaction into two half- reactions. This is best shown by working an example.
For reduction half-reactions, add the electrons to the left side of the equation; for oxidation half-reactions, add the electrons to the right side of the equation. Balance the charge in each half-reaction by adding H + in acidic solution or adding OH - in basic solution. Add H 2 O to balance oxygen and hydrogen.
Worksheets Quiz 1. Oxidation, Reduction, Agents, & Reactions. WS 1. If the answer is no, write a balanced equation for the reaction that would occur. Yes. There is no reaction. 10.
250 Chapter 7 Oxidation-Reduction Reactions 7.1 An Introduction to Oxidation-Reduction Reactions ObjeCtive 2 ObjeCtive 2 Zinc oxide is a white substance used as a pigment in rubber, sun‑blocking ointments, and paint. It is added to plastics to make them less likely to be damaged by ultraviolet radiation and is also used as a dietary supplement.
! 207! Chapter12:!OxidationandReduction.!! Oxidation)reduction(redox)reactions. At!different!times,!oxidation!and!reduction!(redox)!havehaddifferent,but.
Balancing Redox Reactions Worksheet 1 Balance each redox reaction in. acid. solution. Mn 2+ + BiO3 -Æ MnO4 -+ Bi 3+ MnO4 -+ S2O3 2- Æ S4O6 2- + Mn 2+
Answer Key: Assigning Oxidation Numbers. Balancing Redox Reactions Worksheet This page has video solutions for "Practice: Balancing Redox Reactions." Powered by Create your own unique website with customizable templates.
Worksheet 7 - Oxidation/Reduction Reactions Oxidation number rules: Elements have an oxidation number of Group I and II - In addition to the elemental oxidation state of 0, Group I has an oxidation state of +1 and Group II has an oxidation state of +2 Hydrogen -usually +1, except when bonded to Group I or Group II, when it forms hydrides.
General Chemistry I Labs Worksheet 7-1 Redox Reactions Worksheet As you work through the steps in the lab procedures, record your experimental values and the results on this worksheet. Table A: Reactions of Oxidizing Agents Cu 2+ Mg 2 + MnO 1-4 H 2 O 2 KI Question 1: List the oxidizing agents in order, from weakest to strongest.
Oxidation Reduction Worksheet. Determine the oxidation number of each atom in the following substances. NF3 N +3 F -1 K2CO3 K +1 C 4 O -2 c. NO3- N____+5_____ O____-2_____ HIO4 H +1 I +7 O -2 For the following balanced redox reaction answer the following questions. 2 Fe+2(aq) + H2O2(aq) ( 2Fe+3(aq) + 2 OH-1(aq) What is the oxidation state of.
Worksheet 5 balancing redox reactions in acid and basic solution balance each half reaction in basic solution. 2 4 redox reactions notes for the assessed homework test and more exam questions on 2 4 redox reactions go to 2 6 group 2 the alkaline earth metals 2 4 exercise 1 redox reactions answers.
May 08, 2013 · B. reduction, only C. both oxidation and reduction D. neither oxidation nor reduction 23. In the reaction AgNO3(aq)+NaCl(aq) !NaNO3(aq)+AgCl(s), the reactants A. gain electrons, only B. lose electrons, only C. both gain and lose electrons D. neither gain nor lose electrons 24. In the reaction Mg+Cl2!MgCl2, the correct half-reaction for the.
Answers to Chemistry End of Chapter Exercises. 2. (a) oxidation-reduction (addition); (b) acid-base (neutralization); (c) oxidation-reduction (combustion) 4. It is an oxidation-reduction reaction because the oxidation state of the silver changes during the reaction. 6.
Oct 21, 2021 · Redox Reactions. When we consider metabolism, which is the chemical processes of the body, oxidation and reduction reactions are best friends, meaning one cannot exist without the other.Because of.



























0 Response to "35 Worksheet 7 Oxidation Reduction Reactions Answers"
Post a Comment