44 isotopes worksheet high school chemistry
A Periodic Table of the Elements at Los Alamos National ... Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Period Group** 1 IA 1A 18 VIIIA 8A 1 1 H 1.008 2 IIA 2A 13 IIIA 3A 14 IVA 4A 15 VA 5A 16 VIA 6A 17 VIIA 7A 2 He 4.003 2 3 Li 6.941 4 Be 9.012 5 B 10 ... Isotope Notes and HW Key - Dorchester School District Two Chemistry Worksheet NAME: Isotope Notation Block: Complete the table. Recall that A: mass number, Z: atomic number, and ions have charge! Name tsl**/ Atom or ion? Atomic Number Mass Number #of no #of p+ #of e Metal, non- ... Copyriglrt @ 2012 by the Newton South High School Science Department Newton, MA Page2 of2.
PDF Isotopes - Cornell Center for Materials Research Radio isotope decay can also be accelerated by supplying external energy, such as neutrons. Because some radio isotopes generate more neutrons when they decay, this could generate a "nuclear chain reaction" and produce massive energy. This is how the most famous isotope 235U, is used in nuclear power plants and weaponry.

Isotopes worksheet high school chemistry
PDF isotopic abundance practice problems - CHEMISTRY 1. Argon has three naturally occurring isotopes: argon-36, argon-38, and argon-40. Based on argon's reported atomic mass, which isotope exist as the most abundant in nature? Explain.! 2. Copper exists as a mixture of two isotopes. Copper-63 is 69.17% abundant and it has a mass of 62.9296 amu. Unit 1 - MHS Honors Chemistry SC1. Obtain, evaluate, and communicate information about the use of the modern atomic theory and periodic law to explain the characteristics of atoms and elements. a) Evaluate merits and limitations of different models of the atom in relation to the relative size, charge, and position of protons, neutrons, and electrons in the atom. PDF Lesson Plan: Atomic Number, Mass Number, Isotopes and Isotopic Ratios ... This activity will enable your students to calculate the average atomic mass of sample mixtures of isotopes by using various methods. 2. Discuss using an online reading Next, read and discuss "Isotope Analysis" from Harvard University to introduce the occurrence and distribution of oxygen isotopes.
Isotopes worksheet high school chemistry. Mr. Christopherson / Atomic Structure * Isotopes II (40 + 16 slides) * Light (168 slides) * Emission Spectra (14 slides) * Excited State (10 slides) * Hydrogen Spectral Lines (19 slides) * Color (10 slides) * Photoelectric Effect (23 slides) * Extra Slides (21 slides) * Aliens Activity (8 slides) * General * PRINT Atom & PRINT Light Worksheets * Vocabulary: Atomic Structure pdf Isotopes Activity (Chemistry Printable, 6th-12th Grade) Isotopes Activity. Use this enrichment activity about isotopes to develop students' understanding of physical science concepts. Students will interpret a table in this printable in order to answer questions about hydrogen, carbon, and oxygen isotopes. DOC Isotope Practice Worksheet - University of Manitoba Radioactive isotopes emit nuclear radiation in the form of rapidly moving particles or high energy electromagnetic waves. The particles are emitted from the nucleus itself and their removal results in changing the atom from one isotope to another. ... Appendix 3.1-3.4 of the Manitoba Chemistry 30S Curriculum provides excellent isotope ... Nuclear Structure and Isotopes Practice Test - ThoughtCo This ten question practice test will test your knowledge of the structure of atoms, isotopes and monatomic ions. You should be able to assign the correct number of protons, neutrons and electrons to an atom and determine the element associated with these numbers. This test makes frequent use of the notation format ZXQA where: Z = total number ...
Honors Chemistry - Chemistry - Carmel High School Welcome to Mr. Baruch's Web Site. Here you will find information for both Parents and Students. Please feel fee to peruse this site for information regarding HW assignments, quizzes, labs, exams, class events and the course outline. PDF Conejo Valley Unified School District > Homepage What are isotopes? This is just a sampling of what we will address. Throughout this activity you will want to keep both Model 1 and a periodic table handy. —————-—Electron Cloud Nucleus Hydrogen-3 (tritium) Electron cloud Nucleus Carbon-14 26 Electron cloud Nucleus Magnesium-26 Model 1 Symbol Atomic Diacram with Name Isotopes Quiz - Softschools.com To determine the number of neutrons in an atom, subtract the atomic number from the mass number. The proper way to notate an isotope is to use the element's name or symbol with a dash and the element's mass number. Ex: U-238, or Uranium-238. For the following quiz, you will need a periodic table. Select the best answer to the question. PDF Worksheet: Isotope Problems Name - All-in-One High School of 78.92 u while 49.46% of the naturally occurring isotopes of bromine have an atomic mass of 80.92 u. Calculate the average atomic mass of bromine, showing all work: 2. Using the following data, calculate the average atomic mass of magnesium (give your answer to the nearest .01 u) : Show all work! Isotope: 12 24Mg Percent abundance: 78.70% ...
Classwork and Homework Handouts - Penfield High School 25 High School Drive Penfield, NY 14526 (585) 249-6700 fax (585) 248-2810 email info District Home Course Handouts » Chemistry » Unit Sixteen - Nuclear Chemistry » Classwork and Homework Handouts Classwork and Homework Handouts Classwork and Homework Handouts PDF Daigneault Chem.is.try - Home 4. The number determines the name of the atom. 5. The mass number of an atom is the number of number Of in the nucleus of the atom. 6. The isotope notation for nitrogen-15 is as follows: plus the a. The number 15 is the b. The number 7 is the number. number. 15-7=8 c. How many neutrons does nitrogen-15 have? 7. Copy_of_Isotopes_Ions_Atoms_Worksheet_1 - Course Hero Isotopes, Ions, and Atoms Worksheet- Chemistry 1 3 Jason Yeszkonis - Isotopes Ions and Atoms WS-1.pdf 2 Isotopes Ions and Atoms WS notes 2 jeffhogs (39).docx 3 Yoselin Miramontes - Isotopes Ions and Atoms WS-1.pdf 2 Bohr Model Diagrams.docx 2 Molly Myers - IonsIsotopesNew (3).pdf 3 Gay-Lussac's Law Answer Key .pdf 1 PDF Isotope Practice Worksheet 3. Naturally occurring europium (Eu) consists of two isotopes was a mass of 151 and 153. Europium-151 has an abundance of 48.03% and Europium-153 has an abundance of 51.97%. What is the atomic mass of europium? 4. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%),
DOC Isotope Worksheet - Winston-Salem/Forsyth County Schools Write the most common isotope in hyphen notation for the following elements. The most common isotope can be found by rounding the atomic mass found on the periodic table of elements to the nearest whole number. ... Isotope Worksheet Author: WSFCS Workstation Last modified by: WSFCS Workstation Created Date: 9/8/2010 4:01:00 PM Company: WSFCS ...
PDF Isotope Practice Worksheet - Chemistry 3. Naturally occurring europium (Eu) consists of two isotopes was a mass of 151 and 153. Europium-151 has an abundance of 48.03% and Europium-153 has an abundance of 51.97%. What is the atomic mass of europium? Answer: 152.04 amu 4. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%),
PDF Isotopes Worksheet Key - Von Steuben NO, CARBON-12 AND CARBON-13 ARE ISOTOPES OF CARBON BUT HAVE DIFFERENT MASS NUMBERS. 12. Considering your answers to Question 11, work with your group to write a definition of isotope using a sentence. ISOTOPES ARE ATOMS OF THE SAME ELEMENT WITH DIFFERENT MASS NUMBERS. 13. Consult the following list of isotopes symbols: a.
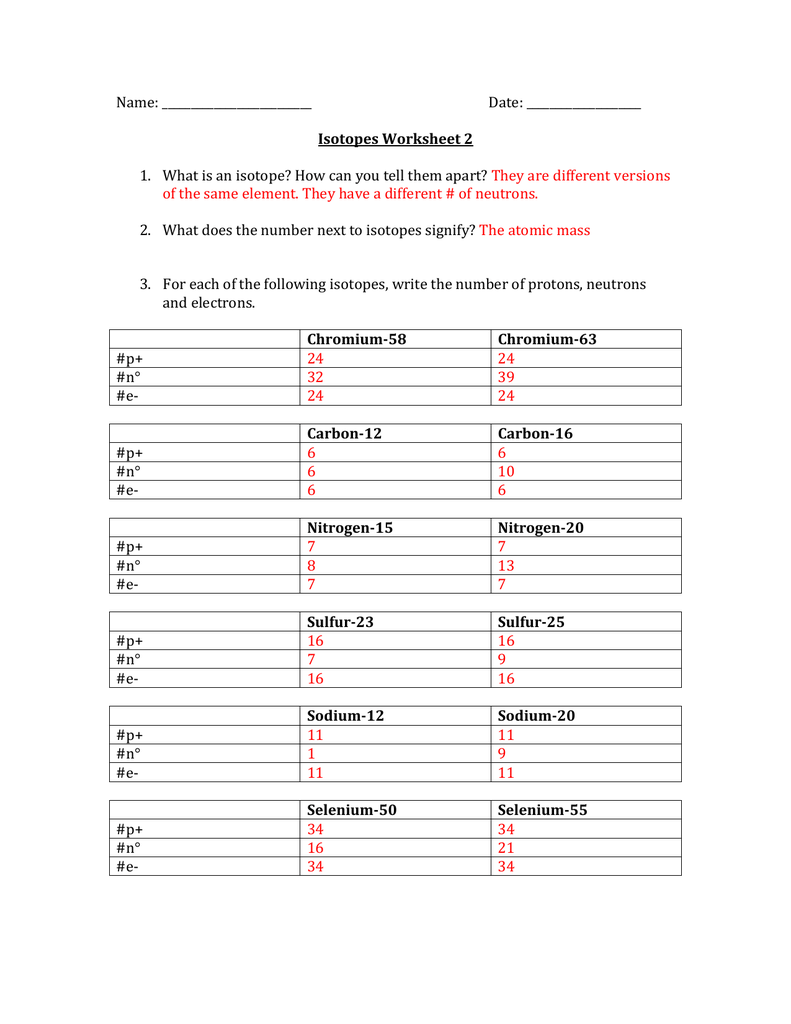
isotopes_worksheet.pdf - Course Hero View isotopes_worksheet.pdf from MATH 123 at Robert F Kennedy Community High School. Name:_ Period:_ Isotopes Worksheet 1. What is an isotope? _ _ 2. What does the number next to isotopes signify? Study Resources. Main Menu; by School ... Rockdale County High School • CHEMISTRY 115. ElementBuilderSE anthony anderson.doc. homework. 5. Bohr.doc ...
radioactive isotope | Description, Uses, & Examples | Britannica radioactive isotope, also called radioisotope, radionuclide, or radioactive nuclide, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess energy by spontaneously emitting radiation in the form of alpha, beta, and gamma rays. A brief treatment of radioactive isotopes follows. For full treatment, see isotope: Radioactive ...
PDF SUBATOMIC PARTICLES and ISOTOPES WORKSHEET 13) An atom with 29 protons and 36 neutrons is an isotope of (A) Si (B) Cu (C) Kr (D) Cl 14) Carbon exists as three naturally occurring isotopes: C-12, C-13 and C-14. As the number of neutrons increase in the isotope, the nuclear charge (A) increases (B) decreases (C) remains the same
An Atoms Worksheet ideal for middle school students Atoms Lessons. Worksheet and Hands-on Learning for Middle School and Up. This is part 2 of our intro to chemistry unit. We've already talked a little about the periodic table and atomic structur e . Today we are moving on to atoms, ions, and isotopes. If you are looking for resources, this is a simple addition to the previous atomic structure ...
Isotopes & Relative Atomic Mass (solutions, examples, videos) More Lessons on Chemistry. The following diagrams show the isotopes of chlorine and how to calculate the relative atomic mass. Scroll down the page for examples and solutions. Isotopes. Isotopes are atoms of the same element that have different number of neutrons. (In order for them to be atoms of the same element, their number of protons would ...
DOC Isotopes Worksheet - Sault Schools Fill in the isotope names and any missing information on the chart. Use your periodic table and the information provided. Assume all atoms are neutral. Mn-42 Mn-40 # of protons 25 25 # of neutrons 17 15 # of electrons 25 25 Ge-62 Ge-64 # of protons 32 32 # of neutrons 30 32 # of electrons 32 32 Pd-94 Pd-97 # of protons
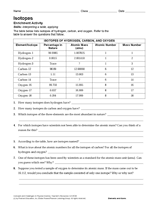
Isotopes Worksheet - Perth Amboy Public Schools 2. What does the number next to isotopes signify? The number indicates the isotope’s mass number. 3. How can you tell isotopes of the same element apart? They will have a different mass number and different number of neutrons. PART II. For each of the following isotopes, write the number of protons, neutrons, and electrons. Assume all atoms ...
Physics Simulations | CK-12 Foundation Isotopes and Nuclear Stability. ... High School Earth and Space Sciences. HS-ESS1-2. HS-ESS1-4. HS-ESS2-4. ... WORKSHEET. Irwin and Ruthie.
What is an Isotope? | Examples, Types & How to Identify an ... Jul 20, 2021 · What is an Isotope? An atom is the smallest unit of matter that contains the properties of a chemical element. This is why atoms are commonly known as the building blocks of chemistry. Atoms are ...
Isotope Activity Teaching Resources | Teachers Pay Teachers This atoms and the periodic table with isotopes digital interactive notebooks are perfect for reviewing parts of an atom, isotopes, atomic number, and atomic mass. Just download it to your drive and then share them with your students using your favorite platform.
Isotopes & Atomic Mass-Inquiry Activity - PhET Contribution Description. This is an inquiry lesson meant to follow Build an Atom activity. Learning Goals: Students will be able to: A.Define "isotope" using mass number, atomic number, number of protons, neutrons and electrons. B.Compare and contrast: element, atom, isotope C.Given the number of protons, neutrons and electrons, find the mass and name ...
PDF Lesson Plan: Atomic Number, Mass Number, Isotopes and Isotopic Ratios ... This activity will enable your students to calculate the average atomic mass of sample mixtures of isotopes by using various methods. 2. Discuss using an online reading Next, read and discuss "Isotope Analysis" from Harvard University to introduce the occurrence and distribution of oxygen isotopes.
Unit 1 - MHS Honors Chemistry SC1. Obtain, evaluate, and communicate information about the use of the modern atomic theory and periodic law to explain the characteristics of atoms and elements. a) Evaluate merits and limitations of different models of the atom in relation to the relative size, charge, and position of protons, neutrons, and electrons in the atom.
PDF isotopic abundance practice problems - CHEMISTRY 1. Argon has three naturally occurring isotopes: argon-36, argon-38, and argon-40. Based on argon's reported atomic mass, which isotope exist as the most abundant in nature? Explain.! 2. Copper exists as a mixture of two isotopes. Copper-63 is 69.17% abundant and it has a mass of 62.9296 amu.












:max_bytes(150000):strip_icc()/Isotope-58dd6b415f9b5846830254ae.jpg)
0 Response to "44 isotopes worksheet high school chemistry"
Post a Comment