40 emission spectra and energy levels worksheet
PDF Emission spectra and energy levels worksheet answer key pdf 2019 2020 Emission spectra and energy levels worksheet answer key pdf 2019 2020 Author: Wuwaka Xebawisaka Subject: Emission spectra and energy levels worksheet answer key pdf 2019 2020. [PubMed] [Google Scholar]24. L., Quantification of electron transfer in liquid-solid contact electri Created Date: 5/7/2022 6:19:49 PM Answers Spectra Emission Worksheet Atomic emission spectra were more proof of the quantized nature of light and led to a new model of the atom based on quantum theory Print exercises and lessons: Hint: For exercises, you can reveal the answers first ("Submit Worksheet") and print the page to have the exercise and the answers practice worksheet with answers For the questions on the blackbody spectrum, use the stellar spectra ...
Lesson Worksheet:Emission and Absorption Spectra | Nagwa Lesson Worksheet: Emission and Absorption Spectra Physics • 9th Grade. Lesson Worksheet: Emission and Absorption Spectra. In this worksheet, we will practice determining the composition of a material from the features that appear in the spectrum of light coming from it. A scientist has a sample of an unknown gas.
Emission spectra and energy levels worksheet
PDF Electron Energy and Light Key energy level 5 to energy level 2. Refer to Models I and 2 for the following questions. a. Label the picture with "n=5 to n=2" and list the corresponding color of light emitted. b. This electron transition (absorbs e eases) ergy. c. This electron moves from a (lower/ gher) ery state to (lower igher) energy state. PDF Emission Spectra - Michigan State University 10.5. In today's lab Figure 10.4: Emission spectra for hydrogen, helium and mercury. is convenient to convert the quantity hc into units of electron volts (eV)2. and nm. Equation 10.1 can now be written as: E = hc λ = 1240 eV nm λ (10.2) where λ is the wavelength in nm and the energy of the photon is in eV. PDF Worksheet 1.3 Emission spectra and electron configurations The emission spectrum of hydrogen is made up of lines in the ultraviolet, visible and infrared regions of the electromagnetic spectrum. Each series of lines follows a distinctive pattern, with lines becoming closer together as their energy increases (wavelength decreases).
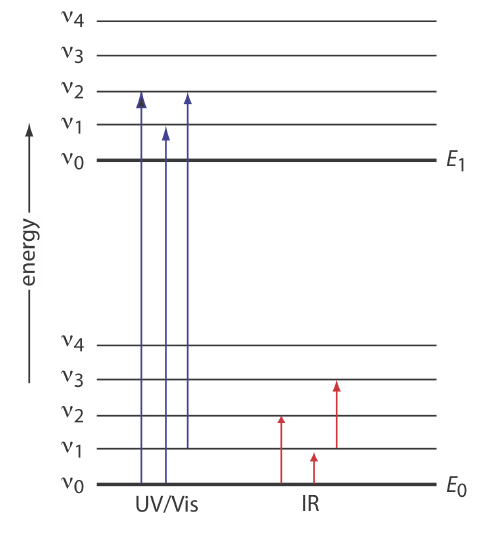
Emission spectra and energy levels worksheet. Quiz & Worksheet - Line Emission Spectra | Study.com Worksheet. 1. Which of the following best describes the reason that line emission spectra contain lines? The energy levels in an atom are discrete and only certain emission wavelengths are ... 2 Emission Spectra Energy Levels and Spectral Charts-1.pdf... View full document. Name: ____________________________________ Period:_________ Page # _____ Emission Spectra and Energy Levels Worksheet Discusion:One convenient method of exciting atoms of an element is to pass an electric current through a sample of the element in the vapor phase. This is the principle behind the spectrum tubes in the demonstration. PPTX Ch5 Modern Atomic Theory - Henry County Schools / Overview The three groups of lines in the hydrogen spectrum correspond to the transition of electrons from higher energy levels to lower energy levels. The Lyman series corresponds to the transition to the n 1 energy level. The Balmer series corresponds to the transition to the n 2 energy level. The Paschen series corresponds to the transition to the n Analyzing Spectra - physics.weber.edu Analyzing Spectra Worksheet. Part 1: Pre-exercise Exercise. An emission spectrum is formed when a _____ gas is viewed directly. An absorption spectrum is formed when a blackbody source (which produces a _____ spectrum) is viewed through a _____ gas. ... Consider just four of the energy levels in a certain atom, as shown in this diagram:
PDF Practical Laboratory #2: Emission Spectra - Michigan State University Equation 2.1 can now be written as: E= hc = 1240 eV nm (2.2) where is the wavelength in nm and the energy of the photon is in eV. 2An electron volt (eV) is the amount of energy it takes to move an electron through one volt of potential so 1 eV = U = qV = (1:6 1019C)(1 V) = 1:6 10 J. 24 .Equipment. Spectra and energy levels | Teaching Resources 5. Intro to quanta using line spectra and LEDs. Lots of background info for the teacher, questions (+answers!), experiment details and images to use. Follows the 'Advancing Physics' scheme, but equally useful for all specifications. Empty reply does not make any sense for the end user. EmissionSpectraEnergyLevelsPractice.pdf - Name Date Pd Emission Spectra ... When visible light energy from a spectrum tube is passed through a diffraction grating, a bright line spectrum, or line-emission spectrum is produced. Each element has its own unique emission spectrum by which it can be identified, analogous to a fingerprint. Such a spectrum consists of a series of bright lines of definite wavelength. Each wavelength can be mathematically related to a definite quantity of energy produced by the PDF Atomic Emission Spectra - Winston-Salem/Forsyth County Schools ery element has a unique atomic emission spectrum, as shown by the examples of mercury (Hg) and strontium (Sr). Classical theory was unable to explain the existence of atomic emission spectra, also known as line-emission spectra. According to classical physics, a ground state atom would be able to absorb any amount of energy rather than only 2
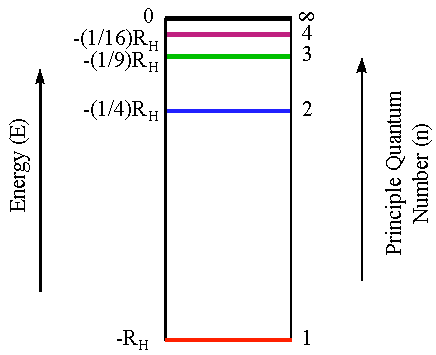
PDF Emission spectra worksheet answers - App Checar following is the width of the emission line from the point where the Is it on one side of the line to where the intensity is 0 on the other side? Give your answer to a Place, put. A0.5 nm b0.8 nm C0.7 nm D0.1 nm e0.4 nm Q7: The figure shows part of the emission spectrum of the hydrogen. The marked emission line is the 656.3 nm emission line on the Answers Worksheet Emission Spectra Search: Emission Spectra Worksheet Answers. Conjunctions Exercises with Answers - Worksheet: Complete the following worksheet on conjunctions Emission spectra and energy levels worksheet answers can be valuable inspiration for those who seek a picture according specific classes you will find it in this site Create your own tracing worksheets with our interactive worksheet maker Download now ... PDF Practice Problems on Emission and Absorption (H atom) Chemistry 121, Mines Energy (10-18 J) 2 3 n-2.179-0.545-0.242-0.136 0 1 4 ∞ 1. Consider the energy level diagram of the hydrogen atom according to the Bohr model (right). (a) Is a photon of light absorbed or emitted when an electron goes from the level n = 4 to n = 3? Give your reasoning. (b) What is the energy of a photon, in J, that is emitted or absorbed when Student Worksheet: Graphing Spectra - NASA We can identify three bright lines for hydrogen in the top spectrum. Measuring from the scale, the wavelengths are 435 nm (purple), 486 nm (blue) and 657 nm (red). Recall (e.g. from the Calculation Investigation) that the the frequency is given by n = c / l, and the energy is given by E = h n (where h = 6.626 x 10-34 J-s, and c = 3 x 10 8 m/s). In the table below we summarize the frequency and energy results for these lines.
Chemistry 101 8-ATOMIC EMISSION SPECTRA excited absorb emit visible. relationship between the energy and the frequency and wavelength is given by the following equation: E final - E initial = ∆E = hυ = hc/λ (2) The quantity h is known as Planck's constant and is equal to 6.626 x 10-34 J ⋅ s (J = joule, s = second). The emission spectrum consists of discrete lines corresponding to the differences in
Emission Worksheet Spectra Answers Spectra Emission Worksheet Answers . jfg.bbs.fi.it; Views: 26599: Published: 10.07.2022 ... This became the accepted theory that electrons could only be in specific locations and these locations corresponded to energy levels Consider the hydrogen spectrum in Model 2 The emission lines correspond to photons of discrete energies that are emitted ...
PDF Emission spectra and energy levels worksheet answers questions pdf answer Emission spectra and energy levels worksheet answers questions pdf answer The tube is filled with a gas. If the photon energy does not correspond to the difference between two energy levels then the photon will not be absorbed (it can still be scattered).
Emission Spectra and Energy Levels Worksheet - Studyres Bright-Line Spectra Objective: To test your ability to analyze bright-line spectra charts Below, bright-line spectral chart for five elements and four unknown samples are given. Answer questions 7-11 based on the information given in the chart. 7. List all elements present in unknown sample W. 8. List all elements present in unknown sample X. 9.

0 Response to "40 emission spectra and energy levels worksheet"
Post a Comment