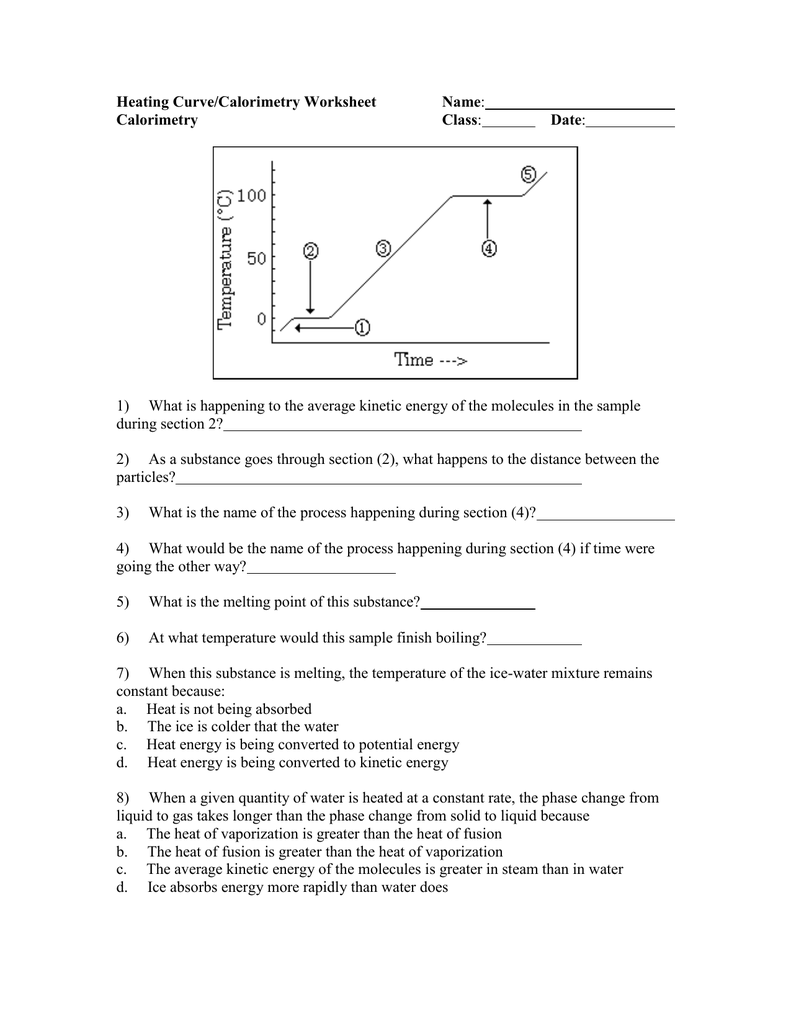
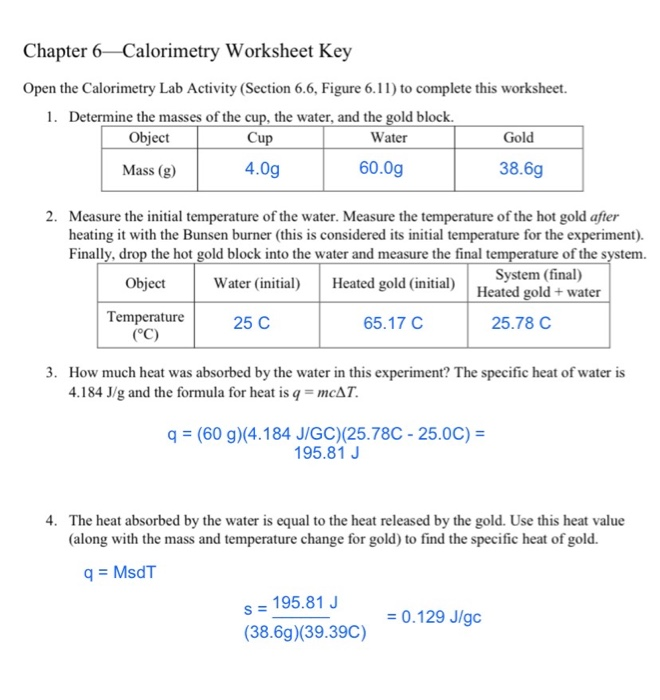
45 calorimetry practice worksheet answers
Calorimetry Worksheet Answers - Kayra Excel Calorimetry practice problems (answers) 1. 119 6 g 120 g rounded answer for sig. Calorimetry Worksheet Answers from briefencounters.ca. Click on the first cell from which to begin the choice. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? If 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter ... PDF Calorimetry and Molar Enthalpy Worksheet Answer Key Calorimetry and Molar Enthalpy Worksheet Answer Key 1) 9.0 grams of charcoal (C) were completely consumed in a bomb calorimeter. If we assume that the 2.0 L of water absorbed all of the heat released by the charcoal, and if the temperature of the water increased from 20.25 ºC to 56.04 ºC, what is the molar enthalpyof carbon? Q water = m. c. T
Honors Chemistry Worksheet - Specific Heat - Quia 4. A piece of food is burned in a calorimeter that contains 200.0 g of water. If the temperature of the water rose from 65.0°F to 105.0°F, how much heat energy was contained in the food? (Ans. 1.87 x 103 J or 4.46 x 103 cal) 5. If the tested food in problem #4 had a mass of 10.0 grams, how many food calories per gram were contained in the food?
Calorimetry practice worksheet answers
DOC Calorimetry Problems 1 Chemistry: Calorimetry Problems 1 Solve the following problems. As always, include work and show the units to ensure full credit. 1. A 445 g sample of ice at -58oC is heated until its temperature reaches -29oC. Find the change in heat content of the system. 2. A 152 g sample of ice at -37oC is heated until it turns into liquid water at 0oC. PDF University of Illinois Urbana-Champaign 3. The heat of combustion of sucrose (C12H24012) in a bomb calorimeter is -3840 kJ/mol. / C12H22011 (S) + Balance the reaction. 02 (g) C02 (g) + 1-120 (g) When 20 grams of sucrose is burned, the temperature of the calorimeter rises by 9. PC. What is the heat capacity (C) of the calorimeter? (molar mass of sucrose = 360.3 g/mol) 5 57 qsys — Calorimetry Practice Problems Worksheet Answers Worksheet by Lucas Kaufmann. Practice Final Exam Problems. The conceal of heat released or absorbed by any chemical process itself be measured using an insulated container called a calorimeter....
Calorimetry practice worksheet answers. DOC Calorimetry Worksheet - Mrs. Gingras' Chemistry Page Explain why this is, based on your knowledge of how bomb calorimetry works. Calorimetry Worksheet. 1) If I burn 0.315 moles of hexane (C6H14) in a bomb calorimeter containing 5.65 liters of water, what's the molar heat of combustion of hexane is the water temperature rises 55.40 C? The heat capacity of water is 4.184 J/g0C. (H = mCp(T (H ... PDF Calorimetry Worksheet W 337 - Everett Community College 2) When 62.3 g of a compound was burned in a bomb calorimeter that contained 0.500 L of water the temperature rise of the water in the calorimeter was 48.00 C. If the heat of combustion of the compound is 1,160 kJ/mol, what is the molar mass of the compound? 0.500 L H 2 O = 500 mL H 2 O = 5.00 x 10 2 g H 2 O H = (5.00 x 102 g H 2 O)(4.184 J/g PDF Calorimetry Problems - Crestwood Local School District Created Date: 2/19/2016 7:32:27 AM PDF II Calorimetry Worksheet - GEOCITIES.ws (6) A 1.567 g sample of naphthalene, C 10H 8 (s), is completely combusted in a bomb calorimeter assembly and a temperature increase of 8.37 0C is observed. When a 1.227 g sample of thymol, C 10H 14O (s) (a preservative and a mold and mildew preventative), is burned in the same calorimeter assembly, the temperature increase is 6.12 0C. If the ...
Heat Capacity Calorimetry Worksheet answers - StuDocu For the same reasoning as question # 1 It takes less energy to raise 1 gram of a material's temperature with a low specific heat, where as 100 J of energy would have less of an effect on a material with a high heat capacity. A 5 g mass of a metal was heated to 100 oC and then plunged into 100 g of water at 24 oC. Calorimetry Worksheet Answer Key - Kayra Excel Calorimetry Worksheet Answer Key. Cal of energy is lost from a 125 g object the. Calorimetry pogil worksheet answer key this is a three activity party. ... Calorimetry practice problems (answers) 1. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the ... PDF Calorimetry Practice Worksheet Calorimetry Practice Worksheet 1) Compound A is burned in a bomb calorimeter that contains 2.50 liters of water. If the combustion of 0.175 moles of thi s compound causes the temperature of the water to rise 45.00C, what is the molar heat of combustion of compound A? DH = mC pDT DH = (2.50 x 103g H 2O)(4.184 J/g 0C)(45.00C) DH = 471,000 J Calorimetry Teaching Resources | Teachers Pay Teachers A printable, 1-page chemistry worksheet with instruction and practice problems. This assignment gives students practice performing enthalpy calorimetry calculations. This uses the heat equation: Q = mc∆T and requires conversion of gram to moles and joules to kilojoules. Students calculate the molar heat of solution (∆H) for a variety of ...
Calorimeter Questions - Practice Questions with Answers ... - Byju's Important Calorimeter Questions with Answers 1) What is a calorimeter? A calorimeter is an apparatus used for calculating the heat developed during a chemical, mechanical or electrical reaction. It also helps to measure the heat capacity of various materials. 2) What is the principle behind a calorimeter? PDF Calorimetry Problems 1 - teachnlearnchem.com 1. A 445 g sample of ice at -58oC is heated until its temperature reaches -29oC. Find the change in heat content of the system. 2. A 152 g sample of ice at -37oC is heated until it turns into liquid water at 0oC. Find the change in heat content of the system. 3. PDF Calorimetry Worksheet - Laney College 6) When a 0.235 g sample of benzoic acid, C 7 H 6 O 2, is combusted in a bomb calorimeter, a 3.62 °C rise in temperature occurs. When a 0.305 g sample of citric acid, C 6 H 8 O 7, is burned, a 1.83 °C temperature rise is observed. If the heat of combustion of benzoic acid is 26.38 kJ/g, what is the molar heat of combustion of citric acid? PDF Name: Period: Seat# Worksheet #3 . Dougherty Valley HS Chemistry Thermochemistry - Calorimetry Practice Mathematical Questions ... incorrect answer. 11) In a coffee-cup calorimeter, 100.0 g of H 2 O and 100.0 mL of HCl are mixed. They both had an initial temperature of 24.6°C. After the reaction, the temperature of both substances is 31.3°C.
PDF Georgetown Independent School District / GISD Home 2. The reaction of zinc with nitric acid was carried out in a calorimeter. This reaction caused the temperature of 72.0 grams of liquid water, within the calorimeter, to raise from 25.00C to 100.0C. Calculate the energy associated with this reaction. Answer: 22, 600J (4.18 22 boo J 3. A 4.00 gram sample of solid gold was heated from 274K to 314K.
PDF Calorimetry Practice Problems Calorimetry Practice Problems (Answers) 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oC? 3135J 3140J (rounded answer for sig. figs.) 2. How many grams of water can be heated from 20.0 oC to 75oC using 12500.0 Joules? 119.6 g 120 g (rounded answer for sig. figs) 3.
Calorimetry Practice Problem Worksheets - K12 Workbook Worksheets are Calorimetry work w 337, Ii calorimetry work, Chemistry work heat and calorimetry problems answers, Calorimetry practice problems answers, Thermochemistry work 1, Calorimeter practice with answers, Calorimetry practice problems with answers, Calorimetry problems with answers. *Click on Open button to open and print to worksheet. 1.
PDF Calorimetry Practice Problems to post - Laney College Calorimetry Practice Problems 1. When 5.000 grams of ammonia react with an excess of oxygen and CH 4 in a bomb calorimeter with a total heat capacity of 15.48 kJ/°C, the temperature of the calorimeter and its contents changes from 19.77°C to 33.90°C. Calculate ∆E and ∆H for the following reaction as written: 2 NH 3 (g) + 3 O 2 (g) + 2 CH ...
PDF Calorimetry Practice Worksheet (Section 17.2) - NOTAPROTON Calorimetry Practice Worksheet (Section 17.2) 1.) Compound A is burned in a bomb calorimeter that contains 2.50 liters of water. If the combustion of 0.175 moles of this compound causes the temperature of the water to rise 45.00C, what is the molar heat of combustion of compound A (in kJ/mole)? The heat capacity of water is 4.18 J/g 0C. ∆H = -q
Calorimetry Worksheets - K12 Workbook Worksheets are Calorimetry work, Calorimetry work w 337, Calorimetry, Calorimetry work, Work calorimetry calorimetry heat capacity q c x, Ii calorimetry work, University of illinois urbana champaign, Mccord calorimetry. *Click on Open button to open and print to worksheet. 1. Calorimetry Worksheet. 2. Calorimetry Worksheet W 337. 3. Calorimetry.
Calorimetry Problems Worksheet Answers - Live Worksheet Online Calorimetry practice problems (answers) 1. The Specific Heat Capacity Of Water Is 4.184 J/G °C. Whenever an adjective is being made use of to validate or describe a noun, it always comes prior to the noun itself, not after. In the laboratory a student uses a coffee cup calorimeter to determine the specific heat of a metal.
Calorimetry Practice Worksheet Answers - 1 - - Gallery Briner Calorimetry Practice Worksheet Answers - 1 -. It shows you how to calculate the quantity of . Temperature of water increases from 20.00 oc to 22.50 oc. A 2.50 g sample of zinc is heated, then placed in a calorimeter containing 65.0 g of water. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by.
Calorimetry Questions and Answers | Study.com Questions and Answers ( 1,861 ) Quizzes (5) A reaction performed in a calorimeter warms 74.0 g of water from 19.5 degree C to 24.7 degree C. Calculate the heat flow for the calorimeter. View ...
PDF Calorimetry Problems Worksheet - bremertonschools.org 6. A 13.5 g sample of gold is heated, then placed in a calorimeter containing 60.0 g of water. Temperature of water increases from 19.00 oC to 20.00 oC. The specific heat of gold is 0.130 J/goC. What was the initial temperature of the gold metal sample? 7. A 28.4 g sample of aluminum is heated to 39.4 oC, then is placed in a calorimeter
Quiz & Worksheet - Calorimetry | Study.com Instructions: Choose an answer and hit 'next'. You will receive your score and answers at the end. question 1 of 3 Express 55.3 J of heat (or energy) in units of calories. 13.2 cal 231.4 cal .076...
Calorimetry Practice Problems Worksheet Answers Worksheet by Lucas Kaufmann. Practice Final Exam Problems. The conceal of heat released or absorbed by any chemical process itself be measured using an insulated container called a calorimeter....
PDF University of Illinois Urbana-Champaign 3. The heat of combustion of sucrose (C12H24012) in a bomb calorimeter is -3840 kJ/mol. / C12H22011 (S) + Balance the reaction. 02 (g) C02 (g) + 1-120 (g) When 20 grams of sucrose is burned, the temperature of the calorimeter rises by 9. PC. What is the heat capacity (C) of the calorimeter? (molar mass of sucrose = 360.3 g/mol) 5 57 qsys —
DOC Calorimetry Problems 1 Chemistry: Calorimetry Problems 1 Solve the following problems. As always, include work and show the units to ensure full credit. 1. A 445 g sample of ice at -58oC is heated until its temperature reaches -29oC. Find the change in heat content of the system. 2. A 152 g sample of ice at -37oC is heated until it turns into liquid water at 0oC.












0 Response to "45 calorimetry practice worksheet answers"
Post a Comment