40 the mole and avogadro's number worksheet
PDF Mole And Avogadros Number Answer Key Avogadro's number and molar mass Key Terms • Mole • Avogadro's''avogadro number amp mole mcqs quiz questions answers june 21st, 2018 - avogadro number and mole mcqs avogadro number and mole test to practice as a mole of any substance contains answer key help with choices as 6 022 10 22''Lab The Mole and Avogadro's Number OpenStudy The Mole And Avogadro S Number Worksheet Answers Resume worksheet template images for the mole and avogadros number worksheet answers and quiz worksheet counting atoms using. The numb er 6 02214179 10 23 is cal led avogadro s number n a or avogadro s constant after the 19th century scientist amedeo avogadro. Mole avogadros number teacher friendly chemistry deanna york.
03- Avogadros Number and the Mole Worksheet_ANSWERS.pdf ... 03- Avogadros Number and the Mole Worksheet_ANSWERS.pdf -. School Carleton University. Course Title CHEM CHEM 1101. Uploaded By SargentRock4225. Pages 1. This preview shows page 1 out of 1 page.

The mole and avogadro's number worksheet
Avogadros Number Worksheets - Teacher Worksheets Showing top 8 worksheets in the category - Avogadros Number. Some of the worksheets displayed are Example exercise atomic mass and avogadros number, Avogadros number practice work, Work mole and avogadros number, Skills work problem solving, Chemistry work name moles molar mass and avogadro, The mole and avogadros number, Mole work, The mole chemistry lesson plan overview day 11. How big is a mole? (Not the animal, the other one ... - TED-Ed The word “mole” suggests a small, furry burrowing animal to many. But in this lesson, we look at the concept of the mole in chemistry. Learn the incredible magnitude of the mole--and how something so big can help us calculate the tiniest particles in the world. DOC AVOGADRO'S NUMBER WORKSHEET - Weebly AVOGADRO'S NUMBER WORKSHEET. Name _____ Date _____ Period _____ 1. How many formula units would there be in a 54.3 gram sample of sodium nitrate? 2. How many atoms of copper are there in 1.00 kg of pure copper? 3. How much would 3.42 X 1023 formula units of mercuric oxide weigh in grams? 4.
The mole and avogadro's number worksheet. Of The Best The Mole And Avogadro Number Worksheet Answers ... The mole and avogadros number worksheet answers 602 x 10 23 individual atomsa value called avogadros number after the chemist amedeo avogadro. 050 moles of 170 moles of KMn04 025 moles of KCI Ecl. Explore fun printable activities for K-8 students covering math ELA science more. Avogadros number is the number of particles in a mole 602 10 23. PDF Chemistry Moles Packet - Chino Valley Unified School District CHEMISTRY WORKSHEET # 3 AVOGADRO'S NUMBER One important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Elements generally exist as the particles we call atoms. PDF Chemistry Worksheet NAME: Moles, Molar Mass, and Avogadro ... Title: Microsoft Word - WS-moles_molar_mass.doc Author: acrosby Created Date: 10/4/2007 8:50:46 PM PPTX Mole PowerPoint - Avogadro's Number, Molar Mass Calculations Key Point #1: The Mole Mole- countingunit standing for 6.02 x 1023 particles Tells us how many particles of a compound are actually involved in a reaction 1 mole = 6.02 x 10 23 particles 602,000,000,000,000,000,000,000 particles Avogadro's Number Mole Samples 1 mole of water = how many particles? 1 mole of sugar = how many particles
PDF Mole And Avogadros Number Answer Key - DAWN Clinic The Mole and Avogadro's Number Worksheet Answers 6.02 x 10 23 individual atoms—a value called Avogadro's Number after the chemist Amedeo Avogadro. The mole is a similar concept to a dozen: one mole of a substance will always have 6.02 x 10 23 atoms or molecules. Atomic weight is a measurement of the mass of each element, and can be easily ... Skills Worksheet Problem Solving - University of Pennsylvania ... Yes; 1.204 2410 is approximately twice Avogadro’s number. Therefore, it is reasonable that this number of atoms would equal about 2 mol. 1 mol Li 6.022 1023 atoms Li 1.204 1024 atoms Li 1.999 mol Li multiply by the inverse of Avogadro's number Number of Li atoms 3 Amount of Li in mol 2 1 Avogadro's number given 1 mol Li atoms Li mol Li 20 Avogadro Number Worksheet Answers | Worksheet From Home 20 Avogadro Number Worksheet Answers. 28 The Mole And Avogadros Number Worksheet Answers avogadros number worksheet answers sch 3u9, moles molar mass and avogadros number worksheet answers, moles and avogadros number worksheet answers, via: starless-suite.blogspot.com. Numbering Worksheets for Kids. Kids are usually introduced to this topic ... Mole Concept- Formula, Explanations, Examples, Related ... Mole Concept- A mole is defined as the amount of a substance that contains exactly the Avogadro number of ‘elementary entities’ of the given substance. The Avogadro number is represented by NA. The Mole Concept is a Convenient Method of Expressing the Amount of a Substance. To Learn more about the Mole Concept with Formulae and Examples with Videos and FAQs, The number of electrons in a ...
Avogadro Number Worksheets - Kiddy Math Avogadro Number - Displaying top 8 worksheets found for this concept.. Some of the worksheets for this concept are Example exercise atomic mass and avogadros number, Work mole and avogadros number, Chem1001 work 4 moles and stoichiometry model 1, Skills work problem solving, Chemistry work name moles molar mass and avogadro, The mole chemistry lesson plan overview day 11, Sch4c unit 2 test ... Discussion worksheet_Week4.docx - Discussion section ... Discussion section worksheet - Week 4 Vocabulary: mole, Avogadro's number, molecular compound, ionic compound, polyatomic ions (name, formula and charge), naming ions, naming molecular compounds, naming ionic compounds Part I: Mole, mass and the number of atoms/molecules conversion Avogadro's number, N A = 6.022 × 10 23 1. What is the amount, in moles, in 0.0355 g of Zn? Class 9 Science CBSE worksheet for Atoms and Molecules The mole is the amount of substance that contains the same number of particles (atoms/ ions/ molecules/ formula units etc.) as there are atoms in exactly 12 g of carbon-12. 2.What is Avogadro's Number? Avogadro and the Mole | Teaching Resources ppt, 2.12 MB. A brief PowerPoint on Avogadro's number, what is a mole and making molar solutions. All images are Creative Comons. Ends with an extension piece on the molarity of water.
Chemistry And Avogadros Number Worksheets - K12 Workbook 1. The Mole and Avogadro's Number 2. Example Exercise 9.1 Atomic Mass and Avogadros Number 3. Avogadros Number Problems Worksheet 4. Avogadro's number practice worksheet 5. Skills Worksheet Problem Solving 6. The Mole Chemistry Lesson Plan Overview Day 11 7. Chemistry mole to mole conversions worksheet 8.
Moles in Chemical Equations: Concept & Use | What is a ... Learn what a mole is, Avogadro's number, and understand mole as a unit of measure. Explore the role and applications of the mole in chemistry and how big it is. Updated: 04/12/2022
Classwork and Homework Handouts - Penfield Central School ... The Mole and Avogadro's Number Worksheet (DOCX 18 KB) The Mole and Volume Worksheet (DOCX 15 KB) Weekly 6 Homework (DOC 52 KB) Weekly 7 Homework (DOC 55 KB) Mole Test - Review Packet (DOCX 18 KB) Mole Test - Review Packet - Answer Key (DOCX 27 KB) Stoichiometry- Mole-Mole Problems Worksheet - Answer Key (DOCX 16 KB)
CHEMISTRY WORKSHEET # 2 MOLE PROBLEMS—THE MOLE AS A UNIT OF MASS CHEMISTRY WORKSHEET # 3 AVOGADRO’S NUMBER. One important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. While a dozen is only 12 particles a . mole is a much larger number—6.02 x 1023 particles. Elements generally exist as the particles we call atoms.
DOCX Mole Conversions Worksheet - Anoka-Hennepin School District 11 Gram to Particle Conversions (two step conversions using molar mass and Avogadro's number) 1. How many oxygen molecules are in 3.36 g of oxygen (O2) [2 x mass of O]? 2. Find the mass in grams of 2.00 x 1023 molecules of F2. 3. Determine the number of molecules of 14 g of nitrogen dioxide (NO2). 4.

Molar Conversion Worksheet / Mole Calculation Worksheet - The sum of the atomic weights of all ...
Molar Mass | Boundless Chemistry - Lumen Learning The bridge between atoms and moles is Avogadro’s number, 6.022×10 23. Avogadro’s number is typically dimensionless, but when it defines the mole, it can be expressed as 6.022×10 23 elementary entities/mol. This form shows the role of Avogadro’s number as a conversion factor between the number of entities and the number of moles.
Lesson: Moles and Avogadro's constant (HT only) | Teacher ... Use 'Mass = Mr x moles' to find any one value given the other two Use Avogadro's constant to calculate number of atoms/molecules in a given mass Calculate the mass of a given number of atoms using the Avogadro constant Intro Quiz Project In Class Presentation Project In Class Transcript Quantitative Chemistry (HT): Relative formula mass (FT only)
PDF The Mole and Avogadro's Number The Mole and Avogadro'sNumber One mole of a substance contains Avogadro's Number (6.02 x1023) of molecules. Directions: How many molecules are in the quantities below? 1) 3.0 moles ANSWER: 1.8 E 24 molecules 2) 2.75 moles ANSWER: 1.66 E 24 molecules 3) 0.82 moles ANSWER: 4.8 E 23 molecules
Mole Worksheet 2 1.docx - Name _ Pd_ The Mole and Avogadro ... View Mole Worksheet 2 1.docx from CHEMISTRY 101 at New Hanover High. Name _ Pd_ The Mole and Avogadro's Number (Section 10.1 & 10.2) One mole of a substance contains Avogadro's Number (6.02 x 1023)
PPT - 1 mole = 6.02 X 10 23 things This is called Avogadro’s number PowerPoint Presentation - ID ...
PDF The Mole and Avogadro's Number - Flagstaff Arts and ... Amoleof objects containsAvogadro's number,6.022 X 1023, objects. Just as a dozen apples is 12 apples, a mole of apples is 6.022 X 1023apples. A mole of iron atoms is 6.022 X 1023iron atoms. A mole of water molecules is 6.022 X 1023water molecules. The NIST 2007 value of Avogadro's number is 6.022 141 79 ± 0.000 000 30 X 1023mol-1.
Diy The Mole Worksheet Answers - Goal keeping intelligence The mole and avogadros number worksheet answers. Chemistry Unit 4 Worksheet 4 Quizlet. The Mega Mole Worksheet 1-10 Convert to Moles 1204 x 1023 atoms He. Mole 10 365 027 moles 16261023 molecules of hcl. 6 It 910 gqg Determine the number of grams in each of the quantities below.
Mole Chemistry Worksheet [NCS0T7] 0 x 10-9 mol/s, find the rate of production of H2 in g/s. Chemistry Mole Worksheets - Kiddy Math Chemistry And Avogadros Number. Download Grams To Moles Worksheet Answers doc. The number of units in a mole also bears the name Avogadro's number, or Avogadro's constant, in honor of the Italian physicist Amedeo Avogadro.

Chemistry: What is the Mole (Avogadro's Number)? 2 practice problems | Homework Tutor - YouTube
Avogadro's Number and the Mole | Introduction to Chemistry Avogadro's number is a proportion that relates molar mass on an atomic scale to physical mass on a human scale. Avogadro's number is defined as the number of elementary particles (molecules, atoms, compounds, etc.) per mole of a substance. It is equal to 6.022×10 23 mol-1 and is expressed as the symbol N A.
Mole-to-Mole Ratios and Calculations of a Chemical Equation ... Nov 17, 2021 · Just to review for a moment, a mole isn't an animal. Well, it is, but not in chemistry. In chemistry, a mole is a unit of measurement, such that one mole of a substance contains 6.022*10 23 particles.
DOC AVOGADRO'S NUMBER WORKSHEET - Weebly AVOGADRO'S NUMBER WORKSHEET. Name _____ Date _____ Period _____ 1. How many formula units would there be in a 54.3 gram sample of sodium nitrate? 2. How many atoms of copper are there in 1.00 kg of pure copper? 3. How much would 3.42 X 1023 formula units of mercuric oxide weigh in grams? 4.




/what-is-a-mole-and-why-are-moles-used-602108-FINAL-CS-01-5b7583f6c9e77c00251d4d68.png)


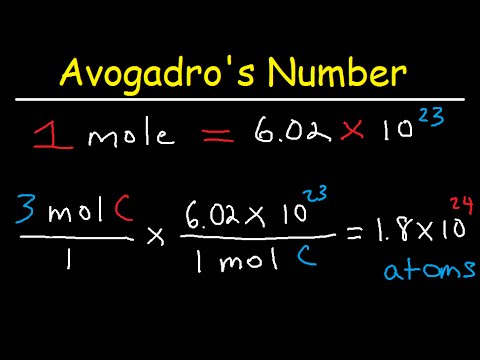
0 Response to "40 the mole and avogadro's number worksheet"
Post a Comment