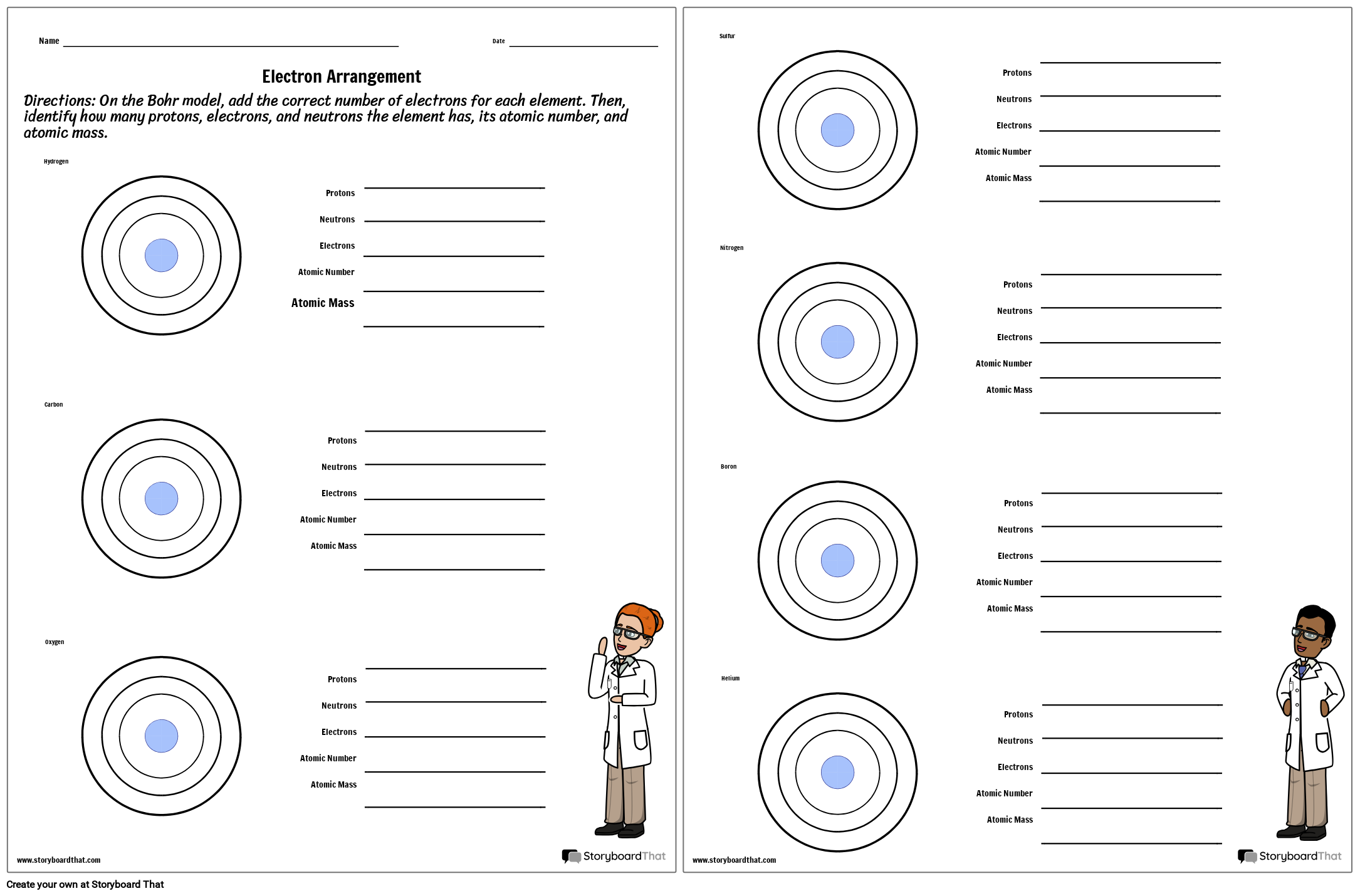
38 atoms protons neutrons electrons worksheet
How to Find Protons, Neutrons and Electrons - Science Struck Neutron: They are neutral subatomic particles found in the center of the nucleus. They were discovered by James Chadwick in 1932. Finding Protons, Neutrons, and Electrons of Isotopes. Isotopes are atoms of the same element with the same proton number, but different number of... Protons, Neutrons, and Electrons Practice Worksheet ...Electrons Practice Worksheet Use the periodic table to find the numbers of protons, neutrons Name of Element Element Symbol Mass Number Atomic Number Protons Neutrons Electrons Atomic Structure Atoms. answers_to_questions_on_pages_100. Name: Period: Atomic Theory...
Protons, neutrons, electrons | Инфоурок The sub-atomic particles: protons, neutrons, electrons. Элементарные частицы: протоны, нейтроны, электроны. exercises Task 2. An atom of an element has 10 neutrons in the nucleus of an atom and the atomic weight of 19.

Atoms protons neutrons electrons worksheet
Protons, Neutrons, and Electrons How Atoms Differ Name: Worksheet: Electron Configurations. I Heart Chemistry! 1. Which electron configuration represents an atom in an excited state? PS-2.1 Compare the subatomic particles (protons, neutrons, electrons) of an atom with regard to mass, location, and charge, and explain how these... Structure of atoms: protons, electrons and neutrons - Free ZIMSEC... Protons and neutrons. The protons are the positive charge in the nucleus of an atom. Electrons have a negative charge. They circle around the nucleus in fixed orbits called electronic shells. The mass of an electron is much smaller than that of a proton. 4.4: The Properties of Protons, Neutrons, and Electrons Because protons and neutrons are so much more massive than electrons, almost all of the mass of any atom comes from the nucleus, which contains all gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in...
Atoms protons neutrons electrons worksheet. How to Find the Number of Protons, Neutrons, and Electrons 1 Calculating Protons, Electrons, and Neutrons. 2 Calculating the Electrons with Ions Present. You need the atomic number to find the amount of protons and/or electrons, unless you Five protons and 6 electrons would give an atom a negative charge, as the number of electrons are more than... Protons Neutrons Electrons: study guides and answers on Quizlet Proton • Neutron • Electron. Protons = +ve (mass 1) Neutrons = neutral (mass 1) Electrons = -ve (mass 1/2000th). Why do atoms usually have no -Neutral (0) charged subatomic particle found in the nucleus of an atom -contributes to the mass of an atom -mass number - protons. electrons. Introduction to the Atom, Protons, Neutrons, Electron, Nucleus, Atomi - Atom - Molecule - Proton - Electron - Neutron - Quark - Nucleus Copyright © 2010 Ryan P. Murphy. 153. Please put the following terms in order from largest to smallest. How to Find the Number of Neutrons, Protons & Electrons for Atoms... The atomic number is equal to the number of protons and electrons for an uncharged atom. The periodic table tells a lot of what you need to know about each element, including the number of electrons, protons and neutrons.
How many protons, neutrons, and electrons does oxygen have? An atom may contain electrons equal to the number of protons in its nucleus, or may contain fewer or greater numbers of electrons than protons, classifying The neutrons and protons are contained within the nucleus, a tight central area of the atom. The electrons, which have a near-zero mass... How to Find Number of Protons, Neutrons, and Electrons Neutrons: Neutrally charged subatomic particles located in the nucleus of an atom. Electrons: Negatively charged subatomic particles located in orbitals surrounding the nucleus. Atomic Mass: A weighted average of the number of neutrons and protons present for all isotopes. Atomic Structure Worksheet - Basic Electricity Take the Atomic Structure (Basic Electricity) worksheet. These questions & answers will help you master the topic! Unless the atom is electrically charged, it will contain 6 electrons as well to balance the charge of the protons. Most carbon atoms contain 6 neutrons, but some may contain more or... Proton, Electron, Neutron - Definition - Formula... - AZ Chemistry No Comments on Proton, Electron, Neutron - Definition - Formula - Application - Worksheet. Atom can be divided into smaller particles consisting of proton, electron, neutron. The basic concept of these theories is really vital to understand the chemistry as a whole study.
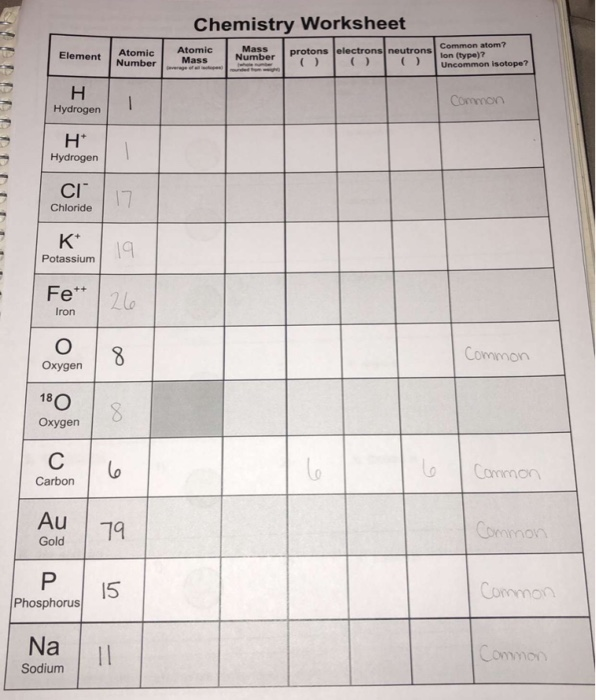
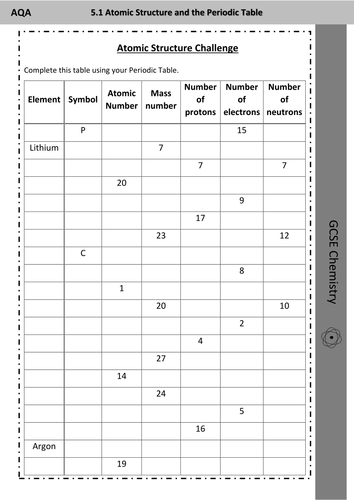
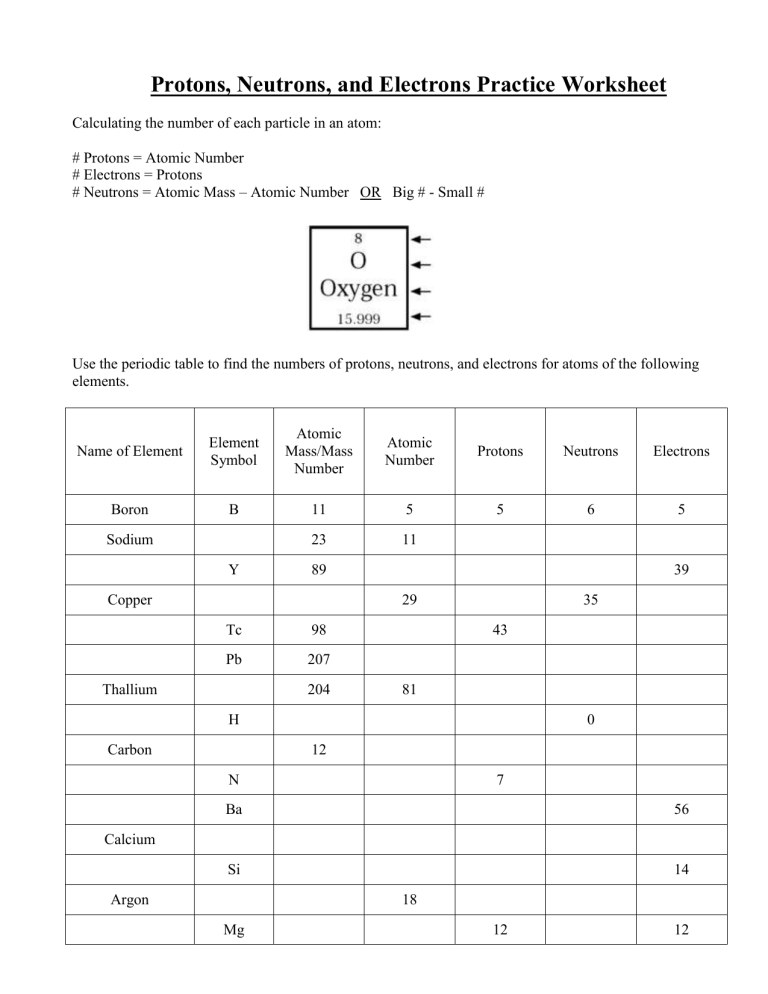
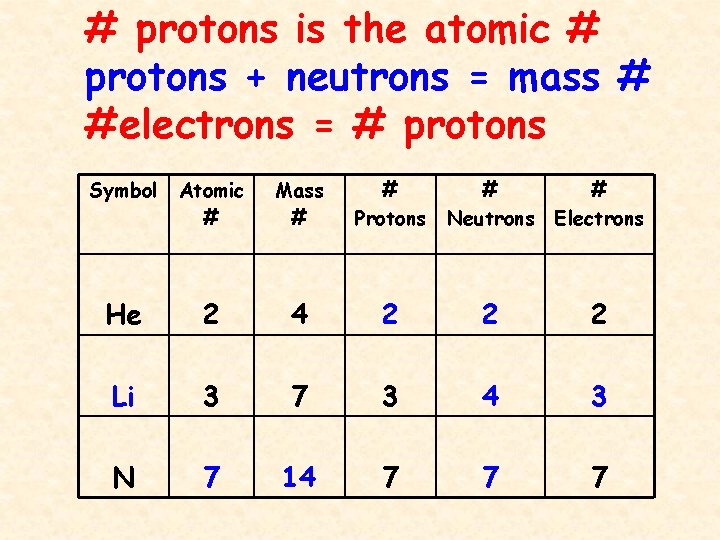
Protons, Neutrons, and Electrons Practice Worksheet Element Practice Atomic Number - Represents both the number of protons and in an element. electrons Most of the mass comes from the To find the number of neutrons, atomic weight from the atomic subtract the mass. 2 Atomic Symbol - a short hand way of writing each elements name. Protons, Neutrons & Electrons | Edexcel GCSE Physics Revision... FREE Physics revision notes on Protons, Neutrons & Electrons. Designed by the teachers at SAVE MY The fundamental charge is equal to the size of the charge on a proton and an electron, however the Although atoms contain particles of different charge, the total charge within an atom is zero. Difference Between Proton, Neutron and Electrons Protons, neutrons, and electrons are commonly called sub-atomic particles. They are essential components for constructing an atom. Each atom has different numbers of protons, neutrons, and electrons. And that is how the atoms preserve their identity and uniqueness. 1.1.2 Atomic Structure • Describe protons, neutrons and electrons. 17 atom - ion symbol protons neutrons electrons charge hydrogen H hydrogen ion + 1 sodium Na sodium ion magnesium magnesium ion + 2 aluminum aluminum ion + 3 nitrogen nitride ion - 3 oxygen oxide - 2 fluorine fluoride -1 Worksheet - Atoms & ions. 18 The arrangement of the electrons The...
72 Best Protons Neutrons Electrons ideas in 2021 | proton neutron... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! atoms, subatomic particles, protons, neutrons, electrons, nucleus, middle school science worksheets, science homework assignments, physical...
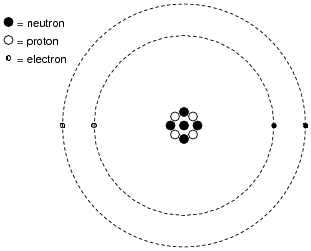
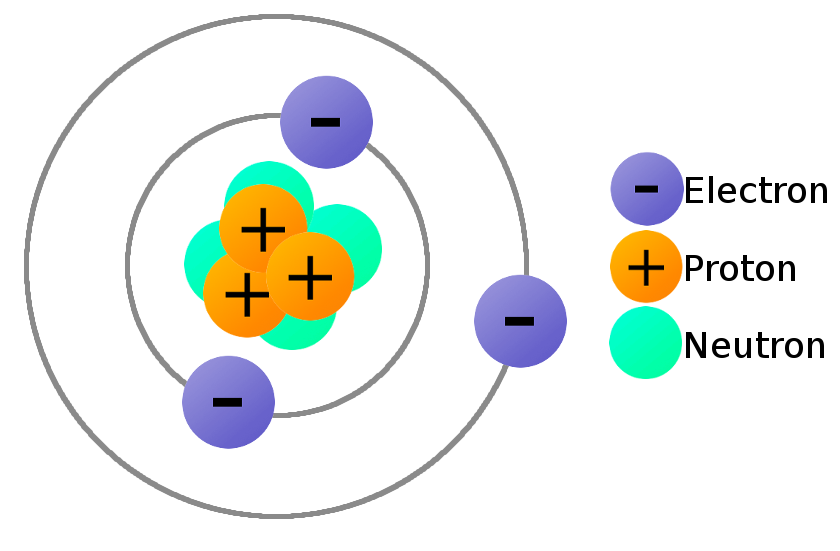
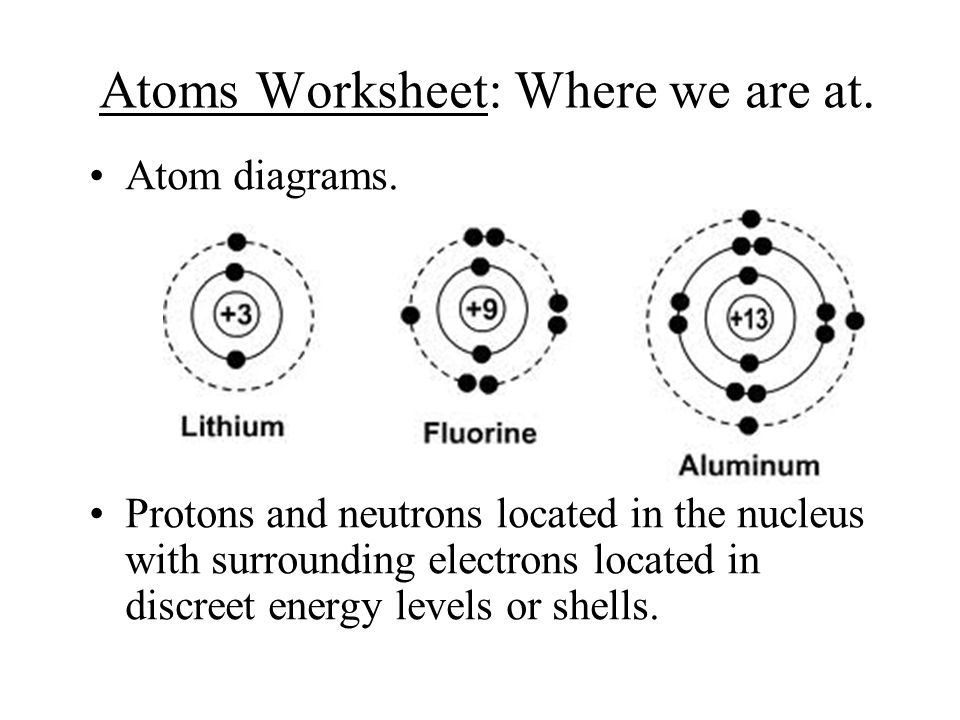
Protons, Neutrons, and Electrons | Middle School Chemistry Proton—positive; electron—negative; neutron—no charge. The charge on the proton and electron are exactly the same size but opposite. The same number of protons and electrons exactly cancel one another in a neutral atom. Note: The picture shows a simple model of the carbon atom.
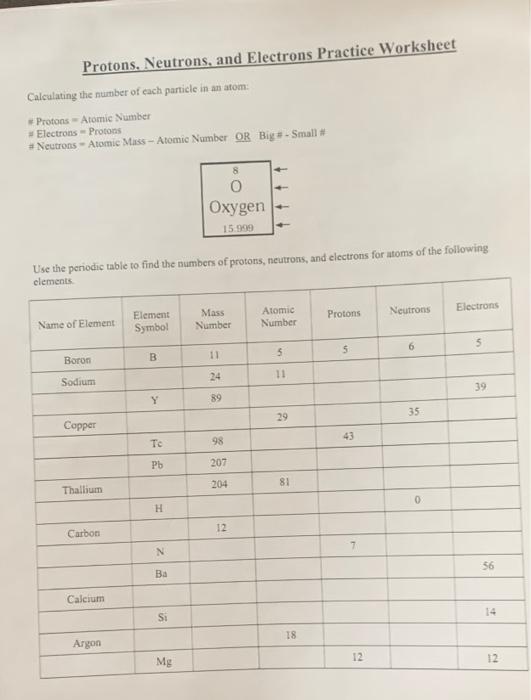
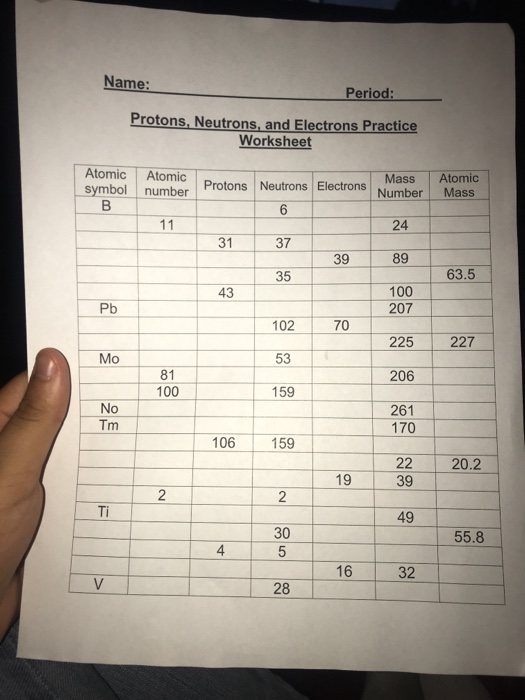
Protons, Neutrons, and Electrons Practice Worksheet -. | Course Hero protons_neutrons_and_electrons_practice_sheet_student_copy. Solutions for the Protons, Neutrons, and Electrons Practice Worksheet: Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass B 5 5 6 5 11 Na 11 11 13 11 24 Ga 31 31 37 31 68 Y 39 39 50 39 89 Cu 29...
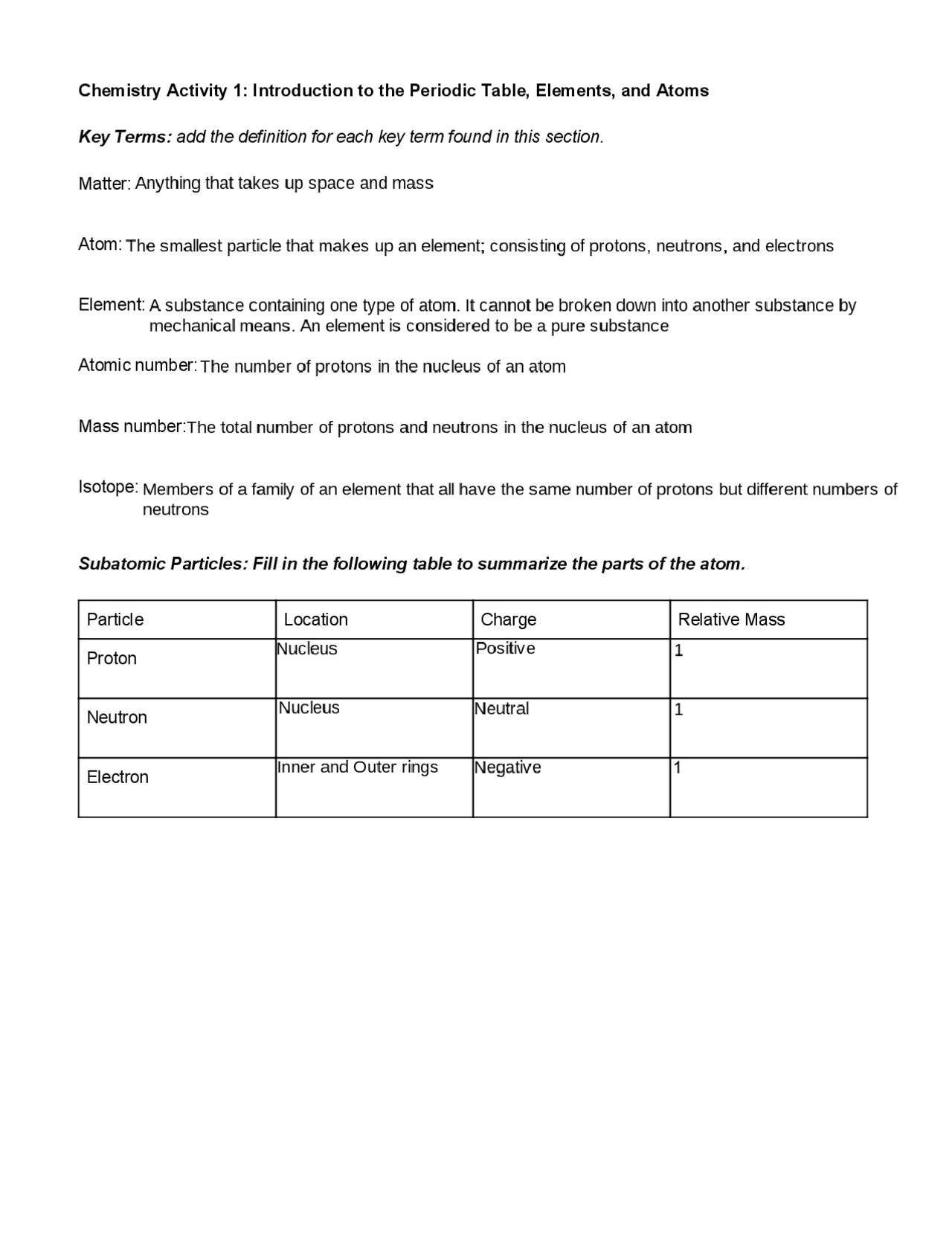
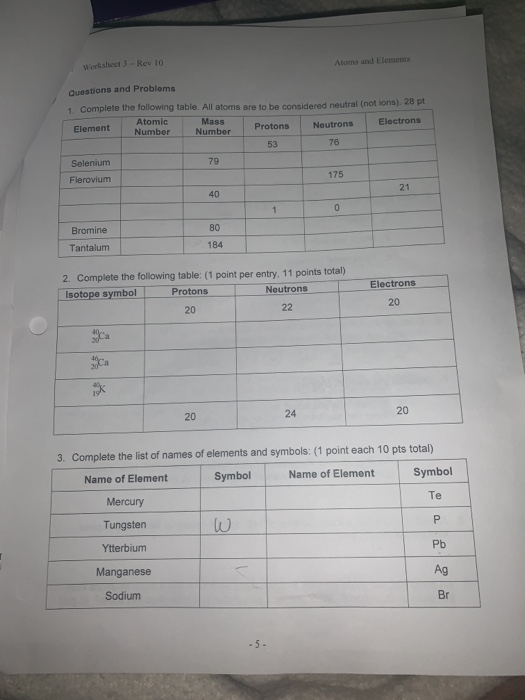
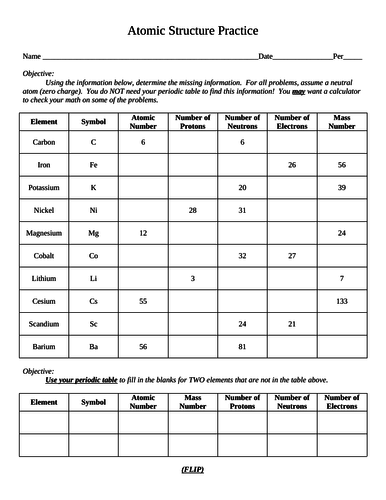
Determining Protons Neutrons and Electrons of Atoms and Ions Mass number: total number of protons and neutrons in the nucleus (not listed on the periodic table, since it varies). NOTE this number is a whole number. Atoms of the same element have the same atomic number, but may have different mass numbers. Isotopic notation for a particular atom (also...
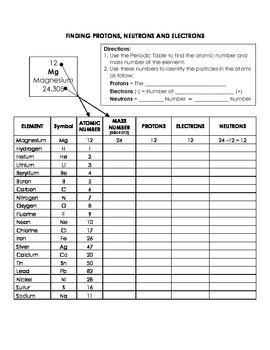
Number of Protons, Neutrons, and Electrons in an Atom A neutral atom has the same number of protons and electrons (charges cancel each other out). If you are given the atomic weight of an atom, you need to subtract the number of neutrons to get the number of protons. Sometimes you can tell the elemental identity of a sample if all you have is the...
2.1 Electrons, Protons, Neutrons, and Atoms - Physical Geology Both protons and neutrons have a mass of 1, while electrons have almost no mass. The element hydrogen has the simplest atoms, each with just one proton and one electron. The proton forms the nucleus, while the electron orbits around it.
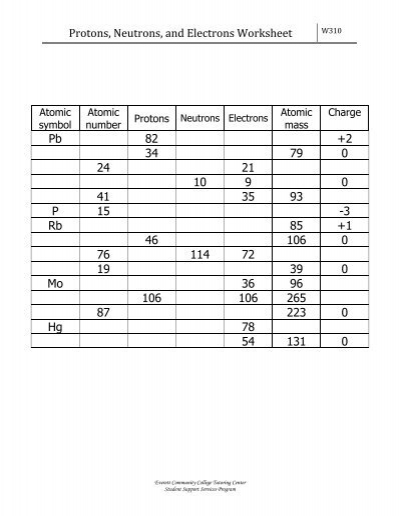
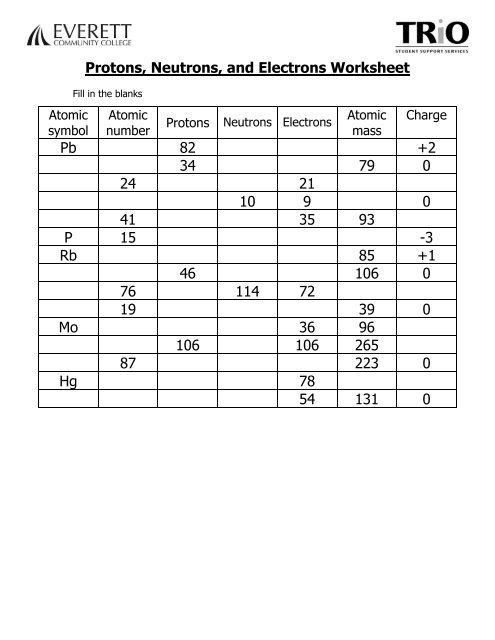
PDF Protons, Neutrons, and Electrons Worksheet 87. Protons. 82 34. 46. 106. Neutrons Electrons. 0. Everett Community College Tutoring Center Student Support Services Program. Protons, Neutrons, and Electrons Worksheet. W310.
Questions and Answers - How do I find the number of protons... Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton must contain 36 electrons since it contains 36 protons. For example, removing an electron from an atom of krypton forms a krypton ion, which is usually written as Kr+. The plus sign means that this is a...
Protons, Neutrons, Electrons worksheet Atoms online worksheet for 9. You can do the exercises online or download the worksheet as pdf. Protons, Neutrons, Electrons identifying the number of subatomic particles. ID: 2428819 Language: English School subject: Physical Science Grade/level: 9 Age: 13-15 Main content: Atoms Other...
Relative Size of: Atoms, Nucleus, Neutrons and Electrons Nucleus and Atomic Diametre. Electrons, Protons And Neutrons. Size Of An Electron. If an electron was the size of a grain of rice, a proton/neutron would be the size of 2000 grains of rice. Electron and Nucleus.
4.4: The Properties of Protons, Neutrons, and Electrons Because protons and neutrons are so much more massive than electrons, almost all of the mass of any atom comes from the nucleus, which contains all gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in...
Structure of atoms: protons, electrons and neutrons - Free ZIMSEC... Protons and neutrons. The protons are the positive charge in the nucleus of an atom. Electrons have a negative charge. They circle around the nucleus in fixed orbits called electronic shells. The mass of an electron is much smaller than that of a proton.
Protons, Neutrons, and Electrons How Atoms Differ Name: Worksheet: Electron Configurations. I Heart Chemistry! 1. Which electron configuration represents an atom in an excited state? PS-2.1 Compare the subatomic particles (protons, neutrons, electrons) of an atom with regard to mass, location, and charge, and explain how these...
















0 Response to "38 atoms protons neutrons electrons worksheet"
Post a Comment