38 12.2 chemical calculations worksheet answers
12.2 Chemical Calculations > 13 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. Mass-Mass Calculations In the laboratory, the amount of ... 12.13, 12.15, 12.19, Assessment 12.2 • Virtual Chemistry Lab 28 12.2 FOCUS Objectives 12.2.1 Construct mole ratios from bal-anced chemical equations and apply these ratios in stoichio-metric calculations. 12.2.2 Calculate stoichiometric quan-tities from balanced chemical equations using units of moles, mass, representative particles,
2.2 mol 2. 1.72 g 6. 0.116 mol 7. 5.2 g 8. 0.071 g 9. 1.44 x 10 24 atoms 10. 7.5 mol 11. 2.0 x 10 22molecules 12. 7.618 x 10 7formula units 13. 0.00498 mol or 4.98 x 10 -3mol 14. 2.53 x 10 19atoms 15. 4.2 x 10 21atoms 16. 1.078 x 10 -6g 17. 15.3 g 18. 1.8 x 10 19molecules 19. 9.8 x 10 19atoms End of preview. Want to read the entire page?
12.2 chemical calculations worksheet answers
2.4 Chemical Formulas - Chemistry Feb 01, 2021 · Posted in Chemistry Worksheets, Science Worksheets. Molecular Geometry Worksheet with Answers admin February 1, 2021. Chemistry (12th Edition) answers to Chapter 12 - Stoichiometry - 12.2 Chemical Calculations - Sample Problem 12.3 - Page 391 11 including work step by step written by community members like you. Textbook Authors: Wilbraham, ISBN-10: 0132525763, ISBN-13: 978-0-13252-576-3, Publisher: Prentice Hall SECTION 12.2 CHEMICAL CALCULATIONS (pages 359-366) This section shows you how to construct mole ratios from balanced chemical equations. It then teaches you how to calculate stoichiometric quantities from balanced chemical equations using units of moles, mass, representative particles, and volumes of gases at STP.
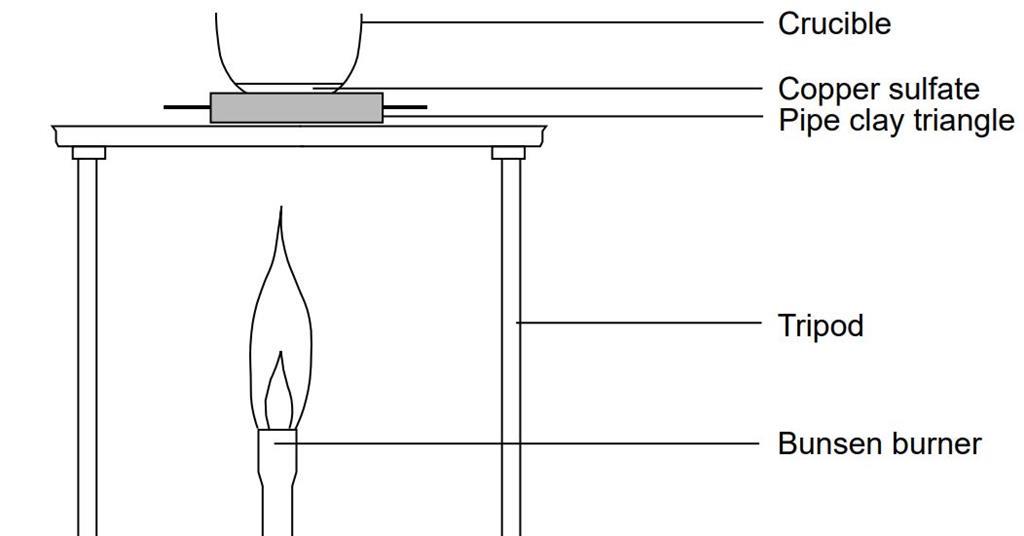
12.2 chemical calculations worksheet answers. Chemistry (12th Edition) answers to Chapter 12 - Stoichiometry - 12 Assessment - Page 412 52 including work step by step written by community members like you. Textbook Authors: Wilbraham, ISBN-10: 0132525763, ISBN-13: 978--13252-576-3, Publisher: Prentice Hall © John Erickson, 2013 Example 2 Fe + 6 HCl → 2 FeCl 3 + 3 H 2 Write three sentences that each describe the ratios in which two of the substances react. 2 atoms of iron react with 6 molecules of hydrochloric acid. For every 2 atoms of iron, 2 formula units of iron (III) chloride are formed. Stoichiometry Practice Worksheet. Balancing Equations and Simple Stoichiometry. Use the following equation to answer questions 8-11: 2 C6H10 + 17 O2 à 12 CO2 + 10 H2O. 13) Using the equation from problem #12, determine the mass of aluminum acetate that can be made if I do this... 17. The substance that restricts the amoun Other reactants used in a chemical reaction is known as the 18. The measured amount or product obtained from a chemical reaction is called the 19. The substance that is not completely used up in a chemical reaction is known 20. If the theoretical yield for a chemical reaction is 100. g and the percentage
Learn 12.2 chemical calculations with free interactive flashcards. Choose from 500 different sets of 12.2 chemical calculations flashcards on Quizlet. Worksheet for Basic Stoichiometry Part 1: Mole ←→ Mass Conversions Convert the following number of moles of chemical into its corresponding mass in grams. 1. 0.436 moles of ammonium chloride 2. 2.360 moles of lead (II) oxide 3. 0.031 moles of aluminium iodide 4. 1.077 moles of magnesium phosphate 5. 0.50 moles of calcium nitrate Chemical Reactions Chapter 12 Study Guide (Unit 9) 2 | P a g e 12.2 Chemical Calculations In chemical calculations, mole ratios are used to convert between moles of reactant and moles of product, or between moles of products. In a typical stoichiometric problem, the given quantity (starting quantity) is first converted to moles. 12.2 Chemical Calculations. STUDY. Flashcards. Learn. Write. Spell. Test. PLAY. Match. Gravity. Created by. esme_o. Terms in this set (12) AT. In mass- mass calculations, the molar mass is used to convert mass to moles. NT. The mole ratio 2 mol HF/1 molSnF2 can be used to determine the mass of SnF2 produced according to the equation: Sn(s)+ 2HF ...
Chemistry (12th Edition) answers to Chapter 12 - Stoichiometry - 12.2 Chemical Calculations - 12.2 Lesson Check - Page 398 22 including work step by step written by community members like you. Textbook Authors: Wilbraham, ISBN-10: 0132525763, ISBN-13: 978--13252-576-3, Publisher: Start studying 12.2 Chemical Calculations. Learn vocabulary, terms, and more with flashcards, games, and other study tools. SECTION 12.2 CHEMICAL CALCULATIONS 1. Calculate the number of moles of hydrogen chloride produced from 10 moles of hydrogen. H 2(g) Cl 2(g) y 2HCl(g) 2. Calculate the number of moles of chlorine needed to form 14 moles of iron(III) chloride. 2Fe(s) 3Cl 2(g) y 2FeCl 3(s) 3. Calculate the number of grams of nitrogen dioxide that are produced from 4.2.1 Write a balanced chemical equation for the formation of Compound A (2) 4.2.2 Determine the percentage purity of the sample of sulphur (6) 4.3. An analysis of compound C shows that this compound contains hydrogen, sulphur and oxygen. Compound C has a molecular mass of 178 g.mol-1 and the following percentage composition:
Chemistry 12 Unit 2 - Chemical Equilibrium Chemistry 12 Worksheet 2-2 ~.J LeChatelier's PrinciIilie Name: KE'i 1. In order to decide what effect a change in total pressure will have on an equilibrium system with gases, what is the first thing you should do when given the balanced equation?
Start studying 12.2 Chemical Calculations. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
May 11, 2013 · 12.2 Chemical Calculations Amounts of reactants and products are always related by mole ratios.Essential Lesson Summary Writing and Using Mole Ratios A mole ratio is a conversion factor derived from the coefficients of a balanced chemical equation. Mole ratios are used to convert between mass and moles in stoichiometric problems.
SECTION 12.2 CHEMICAL CALCULATIONS (pages 359-366) This section shows you how to construct mole ratios from balanced chemical equations. It then teaches you how to calculate stoichiometric quantities from balanced chemical equations using units of moles, mass, representative particles, and volumes of gases at STP.
Chemistry (12th Edition) answers to Chapter 12 - Stoichiometry - 12.2 Chemical Calculations - Sample Problem 12.3 - Page 391 11 including work step by step written by community members like you. Textbook Authors: Wilbraham, ISBN-10: 0132525763, ISBN-13: 978-0-13252-576-3, Publisher: Prentice Hall
2.4 Chemical Formulas - Chemistry Feb 01, 2021 · Posted in Chemistry Worksheets, Science Worksheets. Molecular Geometry Worksheet with Answers admin February 1, 2021.



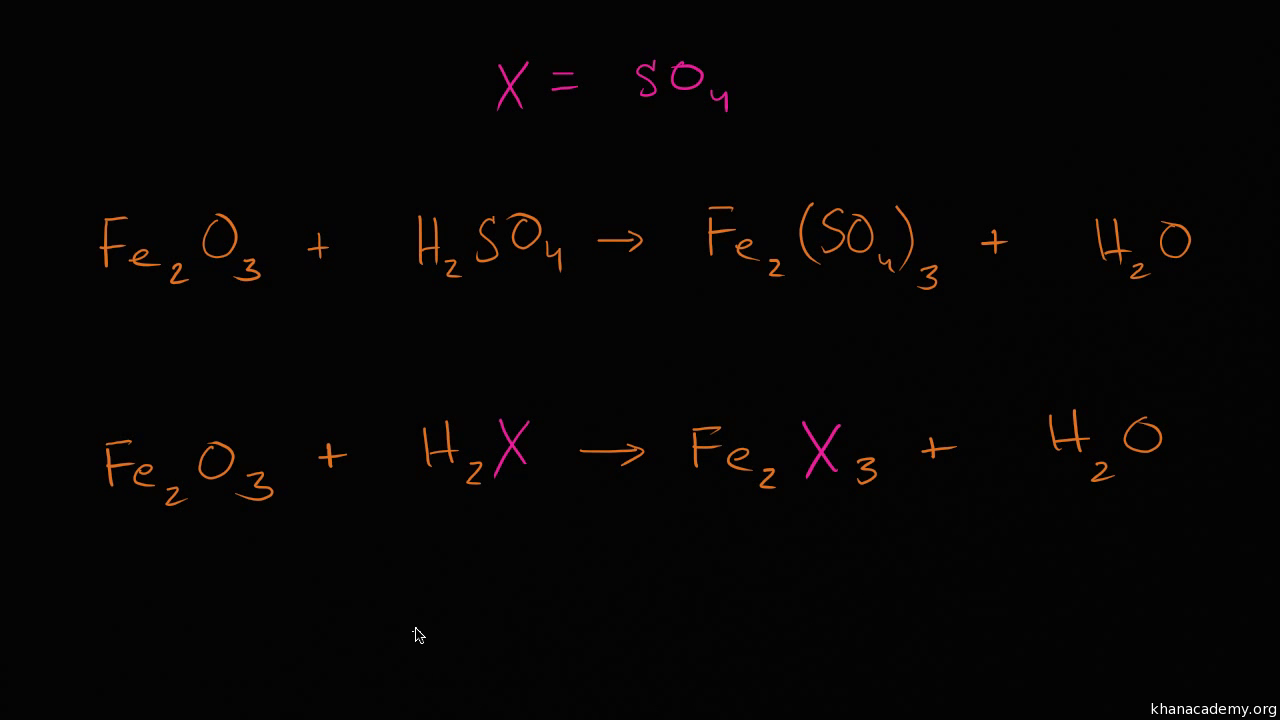




















0 Response to "38 12.2 chemical calculations worksheet answers"
Post a Comment