38 identifying ionic \u0026 covalent bonds worksheet
Identifying ionic and covalent bonds worksheet answers. Chemical formula type of compound. Then use the correct naming rules to write the correct names for each compound. Chapter 9 honors chemistry ionic covalent compound naming first identify whether these compounds are ionic or covalent. Describe the type of bonding that occurs in the compound. Displaying top 8 worksheets found for ionic vs covalent bonds. Snc1d name 1 ionic vs molecular compounds review practice worksheet 1. A molecular compound cannot conduct electricity in any state whereas an ionic compound when dissolved in an aqueous solution can act as a good conductor of electricity. Magnesium chloride e.
Determining the limiting reactant virtual lab answers. Magnesium is the limiting reactant in this experiment. Throughout the lesson, the teacher should emphasize that stoichiometry is important because it allows us to calculate the quantity of each reactant we will need in order to get a desired amount of product. 6/5 from 771 votes. 0925155 which Apr 27, 2021 · fictions to scientific ...
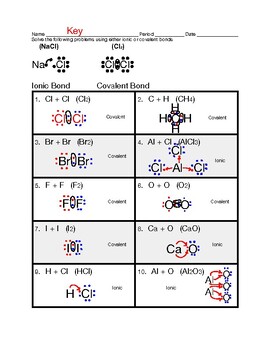
Identifying ionic \u0026 covalent bonds worksheet
Ionic and Covalent Compounds Name: KEY!! 1. We differentiate between two types of compounds: IONIC and COVALENT. ! 2. Ammonia, NH3 is a COMPOUND while nitrogen and hydrogen are _ELEMENTS_. ! 3. In general, molecular compounds form when NONMETALS_combine together. ! 4. In general, ionic compounds form when _METALS & NONMETALS _combine together. ! 5. WORKSHEET: Chemical Bonding - Ionic & Covalent! REMEMBER… Ionic Bond between a Metal and Non-Metal (M + NM) Covalent Bond between a Non-Metal and Non-Metal (NM + NM) PART 1: Determine if the elements in the following compounds are metals or non-metals. Describe the type of bonding that occurs in the compound. Compound Element 1 A. How is a covalent compound different from an ionic compound? Covalent shares electrons where ionic compounds the elements give or receive the electrons. B. Compare a Bohr diagram and a Lewis diagram. Explain how they are: similar. Bohr tell how many electrons in atom, both identify the element. different
Identifying ionic \u0026 covalent bonds worksheet. Naming Ionic Compounds Practice Worksheet Name the following ionic compounds: 1) NH 4 Cl _____ 2) Fe(NO 3) 3 ... Ionic/Covalent Compound Naming Solutions 1) Na 2 CO 3 sodium carbonate 2) P 2 O 5 diphosphorus pentoxide 3) NH 3 ammonia 4) FeSO 4 iron (II) sulfate 5) SiO 2 Naming Ionic and Covalent Compounds Worksheet Answer Key. A Nano-imprinting worksheet that helps you answer questions of nano-chemistry and molecular engineering is a basic part of Ionic-Covalent Worksheets. These worksheets help in creating a workable Nano-imprinting system for use in your nano-engineering work. A) deciding how many electrons are involved in bonding B) deciding if double bonds are present in a molecule C) formulating a statement of the octet rule D) determining the number of single bonds present in a molecule E) predicting the polarity of a bond 83) Which statement about electronegativity is incorrect? Laboratory Worksheet In this activity, you will complete a virtual experiment to identify the unknown compounds. Use the interactive on the assessment page to collect your data. Pre-lab Questions: 1. What are the properties of ionic compounds? It takes a large amount of energy to be able to break an ionic compound, and as a result they have high melting and boiling points.
covalent compounds. Q7. Based on your tests with salt and sugar, compare the ability to conduct electricity in solution of ionic and covalent compounds. Q8. A compound that conducts electricity when dissolved is called an electrolyte. Write a short statement that identifies ionic and covalent compounds as electrolytes or non-electrolytes. Q9. molecule that has polar covalent bonds and engages in hydrogen bonding. Please answer the following questions in preparation for the lab you will be performing: 1. Ionic compounds are generally made up of what kind of elements A metal and a non-metal 2. Covalent compounds are generally made up of what kind of elements Two non-metals 3. Essential Question: How is ionic bonding different from covalent bonding? Comparison of Properties of Ionic and Covalent Compounds Because of the nature of ionic and covalent bonds, the materials produced by those bonds tend to have quite different macroscopic properties. The atoms of covalent materials are bound tightly to each other in stable Ionic & Covalent Compounds Worksheet Write formulas for the following compounds and classify as ionic (I) or covalent (C): lithium chloride I or C ammonium permanganate silver nitrate zinc hydroxide carbon disulfide iron(III) phosphate copper(I) iodide tin(IV) fluoride ...
Identifying ionic bonds and covalent bonds displaying top 8 worksheets found for this concept. Ionic and covalent bonds worksheet. Element of protons of electrons of valence electrons sodium chlorine beryllium fluorine lithium oxygen phosphorus 2. Describe the type of bonding that occurs in the compound. Ionic and Covalent Bonds Worksheet. by. DeCicco Science. $2.00. PDF. Students will demonstrate their knowledge of ionic and covalent bonds. This worksheet requires students to draw the Lewis Dot structures for elements, identify what type of bond will take place, and draw the bond that will take place between them.Students should either circle ... Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu. Based on the characteristics of ionic and covalent bonds, determine if the following is a compound (ionic bond) or a molecule (covalent bond). 1. The substance has a very high melting point and can conduct electricity well. The substance is a compound because it has characteristics of an ionic bond. 2.
2. Identify the elements in each formula 3. Categorize them as either "metals" or "non-metals" 4. Determine the type of bond each compound has. Formula Metals Non-Metals Type of Bond 1. HF Hydrogen Fluorine Covalent 2. NaCl Sodium Chlorine Ionic 3. C 4H 10 4. Al 2O 3 5. CBr 4 6. Na 2S 7. Sr 3N 2 8. H 2S 9. BaF 2 10. C 2H 6 11. NO 2 12 ...
26.11.2021 · Chemistry of life pdf. The books can be downloaded in pdf format. We start the day with Chemistry. 5. nz An chapter-6-the-chemistry-of-life-answer-key 1/2 Downloaded from fall.
29.11.2021 · Ionic Bonding Note November 14 2017 Covalent Bonding Worksheet Ionic Bonding Covalent Bonding 2na s cl 2 g 2nacl s the bonding in sodium. Q. Covalent occurs between non-metallic Oct 29, 2021 · This is a brilliant two page ionic bonding worksheet that is suitable for gcse igcse and myp students. GCSE Past paper Questions a. 1 / 1 electron in outer shell (1) sodium (atoms) lose …
Why are ionic compounds so easy to name? Because most ionic compounds can only form one way, using the oxidation numbers. In covalent compounds, though, non-metals can sometimes com-bine in multiple ways (carbon monoxide; carbon dioxide). So, covalent compounds use prefixes. Hints to remember prefixes : Mono rail - one rail train
6. Covalent compounds often are not soluble in water, have low melting points, and form water solutions that don't conduct electricity. Review 1. Ionic compounds have a much higher melt-ing point than covalent compounds. 2. Compound Property Ionic or covalent A low melting point covalent B molecule as smallest particle covalent C water solution
Unit 6: Ionic and Covalent Bonding. If you are absent, or missed part of the notes, or lost a worksheet or handout, this is the place to come. NOTE: Printing off the notes from here does not excuse you from taking notes or coming to class. Coming to class and taking notes is still the best way to learn and succeed.
1) How are ionic bonds and covalent bonds different, and what types of elements combine to form each? Ionic bonds result from the transfer of electrons from one atom to another (formed by a metal and a non-metal) Covalent bonds result from two atoms sharing electrons (formed by 2 or more non-metals).
Molecular orbital diagram practice worksheet
INTRAmolecular force: holds atoms together in an ionic, covalent or metallic bond. INTERmolecular force: force is BETWEEN molecules or formula units. Identify the types of . intermolecular. forces for the following chemical compounds (HINT: Dipole-Dipole, Electrostatic, Hydrogen or London Dispersion) NaCl : ____ ELECTROSTATIC. CO2: ___ L.D.
Because this nuclide has 26 protons, its atomic number, Z, is 26, identifying the element . The uses of organic compounds impact our lives daily in medicine, agriculture, and general life. The total price includes the item price and a buyer fee. 5. Choose the one alternative that best completes the statement or answers the question. 1) Matter A) has mass. ionic 6. Timberlake [PDF EPUB] Jessy ...
Ionic_Covalent Names: Chapter 9 Honors Chemistry Ionic & Covalent Compound Naming First, identify whether these compounds are ionic or covalent. Then, use the correct naming rules to write the correct names for each compound. Chemical Formula Type of Compound: Ionic or Covalent Compound Name 21) CdBr 2 22) Cr(Cr 2O 7) 3 23) SBr 2 24) (NH 4) 2CrO 4
c) The ΔEN for a covalent bond must have a value > 2.2. FALSE. A covalent bond has a small difference, large differences in electronegativity are ionic bonds. In general a rule of thumb is that ΔEN < 2.1 is considered covalent. 2. For the following compounds identify what has partial positive and partial negative charges. a) HF δ+ H - F δ-
Some of the worksheets for this concept are Chemical bonding, Ionic bonding work 1, 6 chemical bonding, Chemical bonding, Chemical bonding, Types of chemical bonds key, Chemical bonding, Ionic and covalent compounds name key..Science Fair Ideas by Grade and Subject . A property that involves the ability of a substance to react w….
Properties of Ionic Compounds Worksheet 1) Explain why ionic compounds do not conduct electricity in their crystalline form. Electricity can only be conducted when ions are moving. In their crystalline form, the ions in the ionic compound are locked tightly in one place. 2) Why do metals and nonmetals usually form ionic compounds, whereas two ...
WORKSHEET: Ionic vs. Covalent! Ionic Bond between a Metal and Non-Metal (M + NM) Covalent Bond between a Non-Metal and Non-Metal (NM + NM) Determine if the elements in the following compounds are metals or non-metals. Describe the type of bonding that occurs in the compound.
Ionic and Covalent bonding. A unit I have put together for teaching IGCSE. A mixture of my resources and other resources that have been reworked. Any credit has been given in the properties of the file.
Chemistry Worksheet. Naming Compounds & Writing Formulas & Calculating Molar Mass . Questions: 1. Identify the following compounds as Ionic compound or covalent compound, write the name of the
A. How is a covalent compound different from an ionic compound? Covalent shares electrons where ionic compounds the elements give or receive the electrons. B. Compare a Bohr diagram and a Lewis diagram. Explain how they are: similar. Bohr tell how many electrons in atom, both identify the element. different
WORKSHEET: Chemical Bonding - Ionic & Covalent! REMEMBER… Ionic Bond between a Metal and Non-Metal (M + NM) Covalent Bond between a Non-Metal and Non-Metal (NM + NM) PART 1: Determine if the elements in the following compounds are metals or non-metals. Describe the type of bonding that occurs in the compound. Compound Element 1
Ionic and Covalent Compounds Name: KEY!! 1. We differentiate between two types of compounds: IONIC and COVALENT. ! 2. Ammonia, NH3 is a COMPOUND while nitrogen and hydrogen are _ELEMENTS_. ! 3. In general, molecular compounds form when NONMETALS_combine together. ! 4. In general, ionic compounds form when _METALS & NONMETALS _combine together. ! 5.


.png)


0 Response to "38 identifying ionic \u0026 covalent bonds worksheet"
Post a Comment