35 Limiting Reactant Worksheet Answers
Worksheet 14 3 answers to worksheet 14 limiting reagents a limiting reagent is the reactant that is completely used up in a reaction. What is the excess reactant. Consider the reaction i 2o 5 g 5 co g 5 co 2 g i 2 g a 80 0 grams of iodine v oxide i 2o 5 reacts with 28 0 grams of carbon monoxide co. What is the excess reactant. Nov 01, 2021 · Limiting reactant worksheet answers american association of chemistry teachers. Given two reactants in different quantities, the limiting reactant is defined as the reactants that cannot fully convert the given amount of the other reactant to product. Topic 9. residue – solid stopped by the filter during filtration..
Title: HW - limiting reactant practice answers

Limiting reactant worksheet answers
Limiting reactant worksheet answers. Limiting reagent percent yield worksheet answers. All of the questions on this worksheet involve the following reaction. It limits the amount of product produced. The limiting reactant is the first compound to be completely consumed and therefore dictates how much product can be made and how much of. Limiting Reagent Worksheet - Solutions. Using your knowledge of stoichiometry and limiting reagents, answer the following questions: 1) Write the balanced equation for the reaction of lead (II) nitrate with sodium iodide to form sodium nitrate and lead (II) iodide: Pb(NO3)2 (aq) + 2 NaI (aq) ( PbI2 (s) + 2 NaNO3 (aq) In one experiment 0.866 mol of NO is mixed with 0.503 mol of O2. a)Determine the limiting reagent. b) Calculate the number of moles of NO2 produced. This is a limiting reagent problem. Let's calculate the moles of NO2 produced assuming complete reaction for each reactant. 2NO(g) O2(g) ( 2NO2(g)
Limiting reactant worksheet answers. Limiting Reactant and Percent Yield Worksheet.pdf -. School Greenwood High School, Greenwood. Course Title CHEM 521030. Uploaded By ChiefFreedom2380. Pages 2. This preview shows page 1 - 2 out of 2 pages. View full document. For the following equation determine which reactant is the limiting reactant and which reactant is in excess. The amounts of reagent used are shown. Show calculations to support your choices. 3Fe + 4H. 2. O ----> Fe. 3. O. 4 + 4H. 2. 40.0 g 16.0g . 40.0g Fe X . 1molFe 55.8g X 1mol Fe3O4 3molFe = 0.239 mol Fe3O4. 16.0g H2O X . 1molH2O 18.0g . X. Limiting Reactants WS Answers.notebook 3 April 06, 2018 Apr 53:18 PM The fizz produced when an AlkaSeltzer tablet is dissolved in water is due to the reaction between sodium bicarboante, NaHCO3 and citric acid, H3C6H5O7: 3NaHCO3(aq) + H3C6H5O7(aq) 3CO2(g) + 3H2O(l) + Na3C6H5O7(aq) Right here, we have countless books limiting reagent and percent yield worksheet answers and collections to check out. We additionally provide variant types and afterward type of the books to browse. The within acceptable limits book, fiction, history, novel, scientific research, as capably as various other sorts of books are readily nearby here.
Jan 20, 2021 · 49 Limiting And Excess Reactants Worksheet Answers. A) 5 simple steps to successful stoic calculations b) this is the amount of excess reactant actually used in the rxn 2) subtract the answer from step 1 (calculated) from the original amount of excess reactant. Limiting Reagent Worksheet -KEY 1) Write the balanced equation for the reaction given above: CuCl 2 + 2 NaNO 3 Cu(NO 3) 2 + 2 NaCl 2) If 15 grams of copper (II) chloride react with 20 grams of sodium nitrate, how much sodium chloride Limiting Reagents Worksheet from limiting reactant and percent yield worksheet answer key , source:studylib . You need to comprehend how to project cash flow. Regardless of what your company planning goals, cash flow is the most essential resource in the company, and money is the one small business function. b. What is the limiting reactant? c. What is the excess reactant? 2. Use the following BALANCED equation. 2 C 2H 6 + 7 O 2 4 CO 2 + 6 H 2O a. If 15 g of C 2H 6 react with 45 g of O 2, how many grams of water will be produced? ? g H 2 O = 15 g C 2 H 6! 1 mol C 2 H 6 30.0 g C 2 H 6! 6 mol H 2 O 2 mol C 2 H 6! 18.0 g H 2 O 1 mol H 2 O = 27 g H 2 O.
What is the limiting reactant?. Limiting Reagent Worksheet -KEY. All of the questions on this worksheet involve the following reaction: When copper (II) chloride reacts with sodium nitrate, copper (II) nitrate and sodium chloride are formed.... Since the smallest of the two answers is 8.51 grams, this is the quantity of sodium nitrate that ... Worksheet # 3- Adjusting to Reality- Limiting ReactantHow To: Find Limiting Reagent (Easy steps w/practice problem) PHYSICAL SCIENCE - Limiting Reactant and Excess Reactant field and wave electromagnetics cheng solutions, national examination past paper, chapter test reflections social studies, Created Date: 1/27/2016 7:41:57 AM 3. Identify the limiting reactant and determine the mass of CO 2 that can be produced from the reaction of 25.0 g of C 3 H 8 with 75.0 g of O 2 according to the following equation: C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O ANS: 61.9 g 4. How many grams of SO 2 are produced when 152 g of CS 2 react with 48.0 g of O 2 according to the following equation: CS 2
a) Which chemical is the limiting reactant? Zn b) How many grams of ZnS will be formed? 0.3803 mol = 37.1 g c) How many grams of the excess reactant will remain after the reaction is over? 17.7 g 3. Which element is in excess when 3.00 grams of Mg is ignited in 2.20 grams of pure oxygen? O 2 What mass is in excess? 0.226 g O
Limiting reagents and percentage yield worksheet 1. Limiting reactant and percent yield worksheet answers. Ii what percentage yield of iodine was produced. 80 g i2o5 1 mol i2o5 1 mol i2 xs 1 333 8 g i2o5 1 mol i2o5 28 g co 1 mol co. Co g 2 h 2 g ch 3 oh l answer. This reagent is the one that determines the amount of product formed.
Limiting reactant worksheet answers. Limiting reagent percent yield worksheet answers. All of the questions on this worksheet involve the following reaction. It limits the amount of product produced. The limiting reactant is the first compound to be completely consumed and therefore dictates how much product can be made and how much of.
Limiting reactant worksheet answer key free printables. Answers to worksheet 14 limiting reagents a limiting reagent is the reactant that is completely used up in a reaction. A i2o5 5 co 5 co2 i2. Limiting reactant and percent yield practice name 1 consider the following reaction. Answer the questions above assuming we started with 30 grams of.
• Limiting-reactant principle - The maximum amount of product possible from a reaction is determined by the amount of reactant present in the least amount, based on its reaction. To answer this question, calculate the grams of NO 2 needed to react fully with 79.6 grams of NO and 59.5 grams of O 2, by using the balanced equation.
The limiting reagent worksheet is an answer key for chemistry class. If you look up the definition, you will see that it is an instruction sheet which is used to determine the concentrations and the proper amounts of reagents needed to do a particular experiment. Word Equations Worksheet from limiting reagent worksheet answer key with work.
limiting reactant and percent yield practice worksheet answer key, limiting reactant and percent yield worksheet, limiting reactant and percent yield worksheet (show your work), limiting reactant and percent yield worksheet answers, limiting reactant and percent yield worksheet pdf
In one experiment 0.866 mol of NO is mixed with 0.503 mol of O2. a)Determine the limiting reagent. b) Calculate the number of moles of NO2 produced. This is a limiting reagent problem. Let's calculate the moles of NO2 produced assuming complete reaction for each reactant. 2NO(g) O2(g) ( 2NO2(g)
Worksheet 14 3 Answers to Worksheet #14 Limiting Reagents A Limiting Reagent is the reactant that is completely used up in a reaction. This reagent is the one that determines the amount of product formed. Limiting reagent calculations are performed in the same manner as the stoichiometric equations on Worksheet #11. However, with a limiting
Learn how to identify the limiting reactant in a chemical reaction and use this information to calculate the theoretical and percent yields for the reaction. If you're seeing this message, it means we're having trouble loading external resources on our website.
So, if you wish to acquire all these great shots about Limiting Reactant Worksheet Answers, simply click save icon to download the shots to your pc. These are all set for save, if you like and wish to obtain it, simply click save logo on the page, and it will be immediately downloaded in your notebook computer.}
Limiting reagent stoichiometry. Limiting reactant and reaction yields. Worked example: Calculating the amount of product formed from a limiting reactant. Introduction to gravimetric analysis: Volatilization gravimetry. Gravimetric analysis and precipitation gravimetry. 2015 AP Chemistry free response 2a (part 1 of 2)
This worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. A complete answer key is provided at the end. This worksheet can be used in any Chemistry class, regardless of the studen. Subjects:
A which chemical is the limiting reactant. Limiting reactant worksheet answers pdf. This reagent is the one that determines the amount of product formed. In the reaction between co and fe304 the theoretical yield in an experiment is. Answers to practice problems on limiting reactant and yield handout from chapter 4 in chemistry the molecular.
Practice Problems: Limiting Reagents (Answer Key) Take the reaction: NH 3 + O 2 NO + H 2 O. In an experiment, 3.25 g of NH 3 are allowed to react with 3.50 g of O 2.. a. Which reactant is the limiting reagent?
Let's Get Started: Answer Key 2. Under normal conditions, C 3 H 8 would be the limiting reactant because there is a very large amount of oxygen in any room. You rarely run out of oxygen when you burn something, and this reaction is a combustion reaction. You could make oxygen the limiting reactant (like Question
Limiting Reagents and Percentage Yield Worksheet Author: SPANG Last modified by: Jewell K. Whitaker Created Date: 8/10/2016 8:59:00 PM Company: hempfieldarea school district Other titles: Limiting Reagents and Percentage Yield Worksheet
Limiting Reagent Worksheet - Solutions. Using your knowledge of stoichiometry and limiting reagents, answer the following questions: 1) Write the balanced equation for the reaction of lead (II) nitrate with sodium iodide to form sodium nitrate and lead (II) iodide: Pb(NO3)2 (aq) + 2 NaI (aq) ( PbI2 (s) + 2 NaNO3 (aq)
Limiting Reagent Worksheet #1 1. Given the following reaction: (Balance the equation first!) C 3H 8 + O 2-----> CO 2 + H 2O a) If you start with 14.8 g of C 3H 8 and 3.44 g of O 2, determine the limiting reagent b) determine the number of moles of carbon dioxide produced c) determine the number of grams of H 2O produced
Answer the questions at the top of this sheet, assuming we start with 100 grams of calcium carbonate and 45 grams of iron (III) phosphate. Limiting Reagent Worksheet Answers. For the following reactions, find the following: a) Which of the reagents is the limiting reagent? b) What is the maximum amount of each product that can be formed?
Limiting reactant worksheet answers along with fresh limiting reactant worksheet fresh percent yield and limiting. What is the limiting reactant. 0 3803 mol 37 1 g c how many grams of the excess reactant will remain after the reaction is over. Limiting excess reagents 1. Limiting reactant worksheet answers. Limiting reagent worksheet key.
Acces PDF Limiting Reagent And Percent Yield Worksheet Answers Limiting Reagent And Percent Yield Worksheet Answers Right here, we have countless ebook limiting reagent and percent yield worksheet answers and collections to check out. We additionally meet the expense of variant types and then type of the books to browse.
Limiting Reactant Worksheet Answers. Worksheet February 01, 2019 03:29. If both reactants are found in, the reactant that's left over after the reaction is complete is known as the surplus reactant. The leftover reactants are known as the additional reactants. A limiting reactant is a reagent that's completely consumed throughout a chemical.
Use the steps below to solve the following problem to determine the limiting reactant. 1. Write a balanced equation. 2. Do a separate mass to mass problem starting with each reactant. The smaller answer is correct. To find out how much of the excess reactant is left over, 1. Start with the initial mass of the limiting reactant and 2.
The reactant used up first is known as the limiting reactant. The other reactants are partially consumed where the remaining amount is considered "in excess". This example problem demonstrates a method to determine the limiting reactant of a chemical reaction. Limiting Reagents Worksheet - New Paltz Middle School This is a limiting reagent problem.








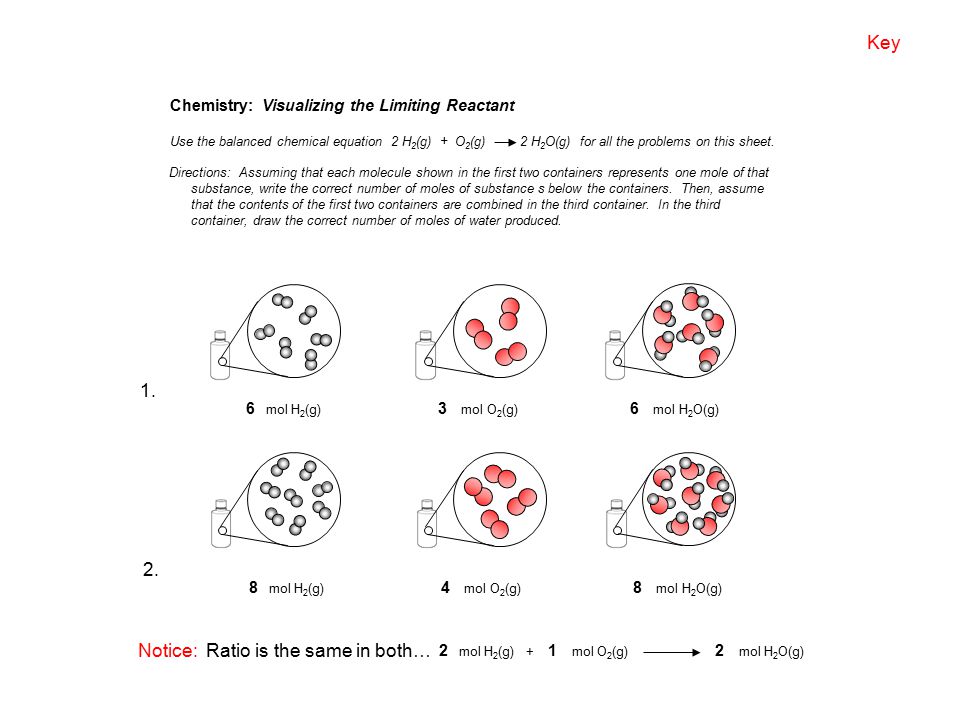





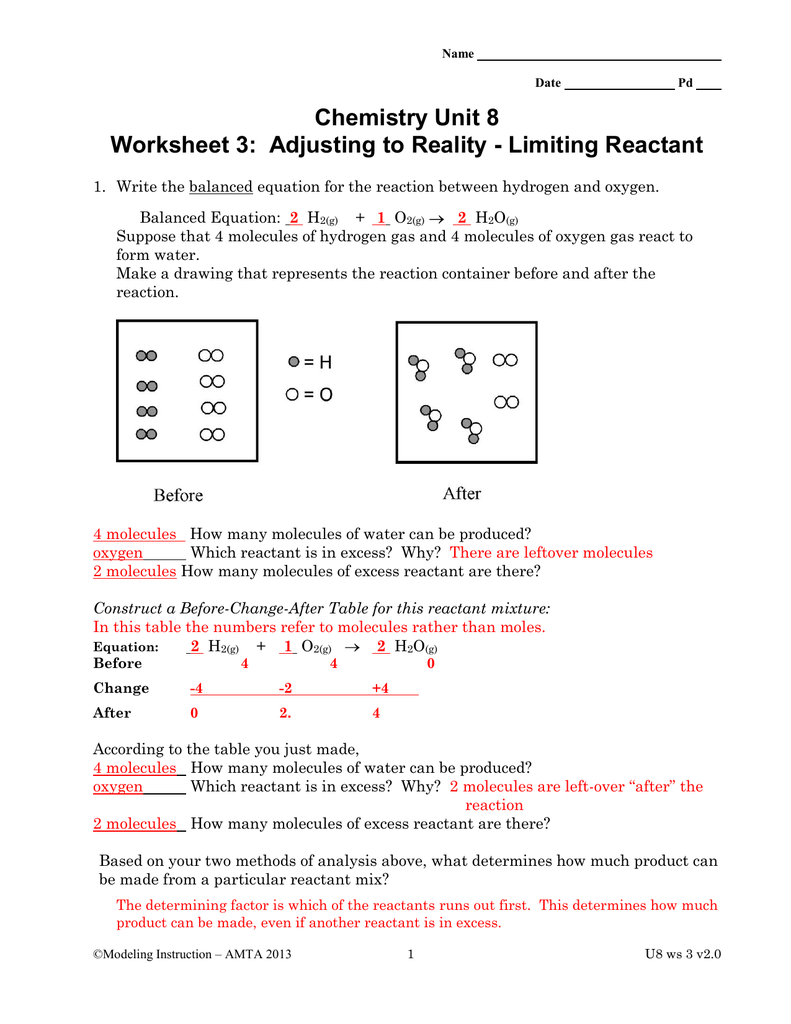









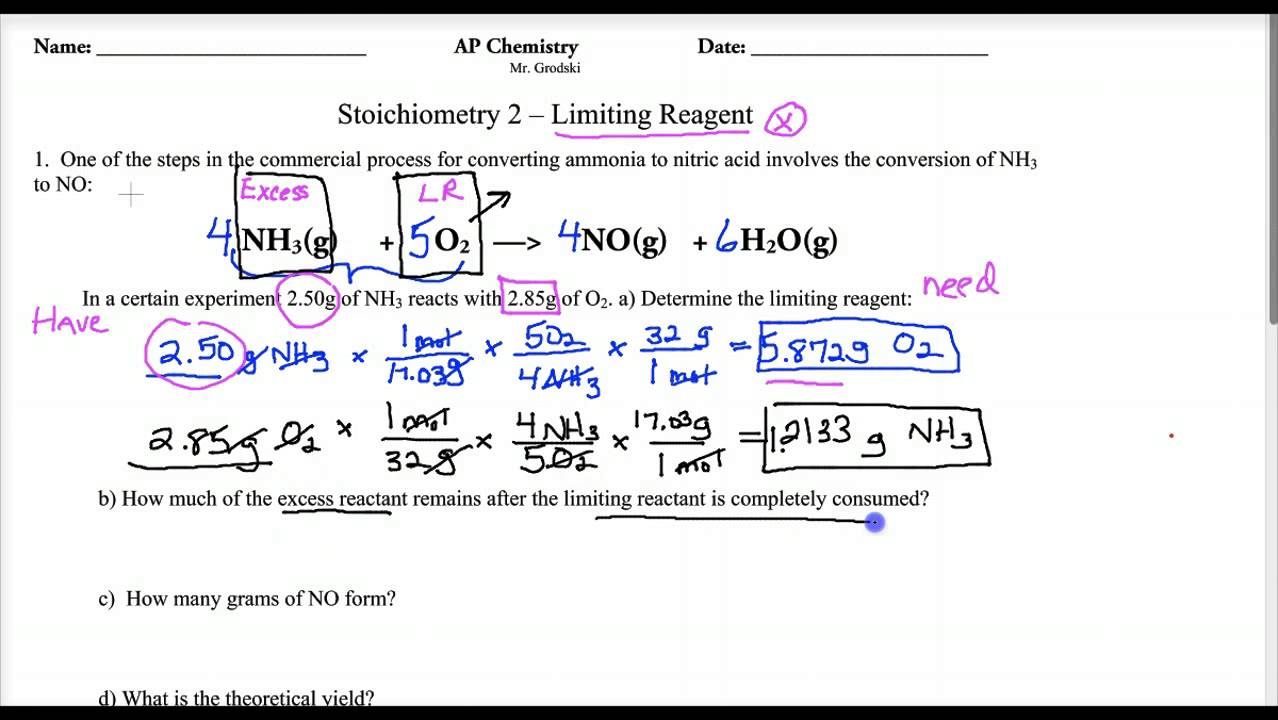

0 Response to "35 Limiting Reactant Worksheet Answers"
Post a Comment