34 Molecular Mass And Mole Calculations Worksheet Answers
A mole is like a dozen. It is a name for a specific number of things. There are 12 things in a dozen, and 602 hexillion things in a mole. We'll talk about wh... Nov 24, 2019 · The units of mass are typically grams. Mass percent is also known as percent by weight or w/w%. The molar mass is the sum of the masses of all the atoms in one mole of the compound. The sum of all the mass percentages should add up to 100%. Watch for rounding errors in the last significant figure to make sure all the percentages add up.
The mole - Formula mass and mole calculations - GCSE The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula. Moles are units used to measure substance amount. 3.1 Formula Mass and the Mole Concept - Chemistry 2e The molar mass of an element (or compound) is the.

Molecular mass and mole calculations worksheet answers
2. convert grams of each into moles of each by dividing by molar mass 3. divide the moles of water by the moles of the anhydrous to determine the number of moles of water in the formula 4. write the formula of the anhydrous followed by a dot then the # of moles of water and "H 2O" Example: CuSO4 · 5H 2 O Naming a Hydrate 1. Mole Calculation Worksheet - Answer Key Grams Moles And Molecular Mass Worksheet Answer Key The molar mass of Cl 2 (which we get from the atomic mass of Cl from the periodic table) is 70.90 Page 3/5. Bookmark File PDF Mass And Moles Worksheet Answers g/mol. We must invert this fraction so that the units cancel properly:. Molar mass can be measured by a number of experimental methods, many of which will be introduced in later chapters of this text. Molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. As the name suggests, an empirical formula mass is the sum of the average atomic masses of all the.
Molecular mass and mole calculations worksheet answers. Mole Calculation Worksheet - Answer Key Grams Moles And Molecular Mass Worksheet Answer Key The molar mass of Cl 2 (which we get from the atomic mass of Cl from the periodic table) is 70.90 Page 3/5. Bookmark File PDF Mass And Moles Worksheet Answers g/mol. We must invert this fraction so that the units cancel properly:. Sep 23, 2021 · Collectively, these conversions are called mole-mass calculations. As an example, consider the balanced chemical equation \[Fe_2O_3 + 3SO_3 \rightarrow Fe_2(SO_4)_3\] If we have 3.59 mol of Fe 2 O 3, how many grams of SO 3 can react with it? Using the mole-mass calculation sequence, we can determine the required mass of SO 3 in two steps. Read Free Empirical And Molecular Formula Worksheet Answers 6 1. And a molar mass of 88 grams per mole. James Holler Stanley R. The empirical formula for the compound having the formula H2C2O4 is A C2H2 B CO2H C COH D C2O4H2 E COH2 2. Show work on a separate sheet of paper. Molecular Mass Percent Composition Worksheet Answers Empirical Formulas. Exercise 1.2 Mole calculations This exercise will familiarise you with some basic calculations using the mole concept. moles = mass g m ola rm as s in i nm ol dm. In part (a) remember to use the mole ratio. In part (a)(iv) you need to rearrange the equation: (mass (in g) = moles × molar mass (in mol dm 3). a Lead oxide, Pb 3 O 4, is reduced by.
Chemistry Mole. In chemistry, the mole, also called Avogadro's Number, is a unit that is useful in converting between atomic mass and molar mass. One mole is 6.02 x 10 23 of something, which was derived from the number of atoms in 12 grams of carbon-12. The meaning and functionality of Avogadro's number in chemistry. YouTube. The definition of Relative Molecular Mass Mr (also referred to as Molar Mass) is The mass of a single molecule on a scale on which the mass of an atom of carbon — 12 has a mass of 12 atomic mass units. Relative Molecular Mass of a molecule is calculated by adding together the relative atomic masses of the atoms in the chemical formulae. ID: 1216466 Language: English School subject: Chemistry Grade/level: 8 Age: 12-14 Main content: Molecular mass Other contents: Add to my workbooks (2) Download file pdf Embed in my website or blog Add to Google Classroom the molecular formula of the compound given that the molar mass is 237 g/mol. cCQ 12.01 B (MA 34 937 = a.53$_ 35. 14. A compound has a molar mass of 86 g/mol and has the percent composition (by mass) of 55.8% C, 37.2% O, and 7.0% H. Determine the empirical formula and the molecular formula. 7, H 233 2,33 2.33 (0/93 2.33
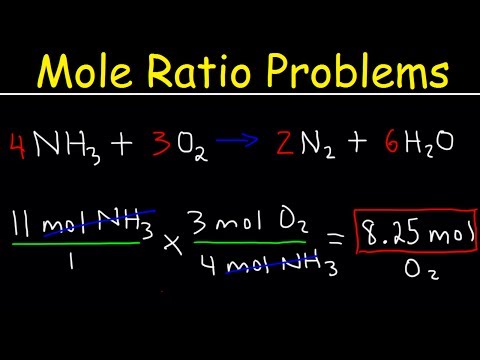
Molecular mass and percent composition worksheet answers. Express answers to two. If its gram molecular mass is 102 gmole what is its molecular formula. Atomic mass values for individual elements can be obtained from our periodic table of the elements. A compound with an empirical formula of c2h8n and a molar mass of 46 grams per mole. ole Calculation Worksheet - Answer Key What are the molecular weights of the following compounds? 1) NaOH 22.99 + 16.00 + 1.01 = 40.00 grams/mol 2) H3PO4 3(1.01) + 30.97 + 4(16.00) = 98.00 grams 3) H2O 2(1.01) + 16.00 = 18.02 grams 4) Mn2Se7 2(54.94) + 7(78.96) = 662.60 grams 5) MgCl2 = 24.31 + 2(35.45) = 95.21 grams 6) (NH4)2SO4 2(14.01) + 8(1.01) + 32.07 + 4(16.00) =132.17 grams Solve the. Mole Calculations Review Worksheet – answers on next page. 1. Calculate the molar mass of each compound. a. LiOH c. Mg(C 2H 3O 2) 2. b. barium bromide d. Ca(NO 3) 2. 2. How many molecules are in 45.0 grams CH 4? 3. How many moles are in 18.8 grams NaOH? 4. A salt 23shaker containing 9.58 x 10 formula units NaCl contains how many moles? 5. There are three definitions (equalities) of mole. They are: 1 mole = 6.02 x 1023 particles 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 L of a gas at STP (You do not need to worry about this yet) Each definition can be written as a set of two conversion factors. They are:
mole of a compound contains Avogadro's number (6.022 x 1023) of molecules (molecular compound) or formula units (ionic compound). The molar mass of a compound tells you the mass of 1 mole of that substance. In other words, it tells you the number of grams per mole of a compound. The units for molar mass are, therefore, grams/mole. To find the.
www.njctl Chemistry Mole Calculations Molar Mass 19)Which of the following is not a true statement concerning the gram atomic mass? A) The gram atomic mass is the number of grams of an element that is numerically equal to the atomic mass in amu. B) The gram atomic mass is 12 g for magnesium. C) The gram atomic mass is the mass of one mole of atoms.
Aug 01, 2019 · The molecular formula of a compound is a representation of the number and type of elements present in one molecular unit of the compound. This 10-question practice test deals with finding the molecular formula of chemical compounds.. A periodic table will be required to complete this test. Answers appear after the final question.
6) Determine the volume of a substance (at STP) when given its mass, the number of particles present, or the number of moles and vice versa. 7) Determine the empirical and/or molecular formula of a substance when provided key information.
2. convert grams of each into moles of each by dividing by molar mass 3. divide the moles of water by the moles of the anhydrous to determine the number of moles of water in the formula 4. write the formula of the anhydrous followed by a dot then the # of moles of water and "H 2O" Example: CuSO4 · 5H 2 O Naming a Hydrate 1.
Mole to Grams, Grams to Moles Conversions Worksheet. What are the molecular mass of the following compounds? 1) NaOH 2) H3PO4 3) H2O 4) Mn2Se7. 5) MgCl2 6) (NH4)2SO4. There are three definitions (equalities) of mole. They are: 1. mole = 6.02 x 1023 particles. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass)
Moles Number: A mole is an amount of a substance equal to a quanity of 602 sextillion particles (atoms, molecules, or unit cells). Written as 602,000,000,000,000,000,000,000 = 6.02 x 1023 Mass: A mole is the atomic mass (element), molecular mass
Mole Calculations I. We apply the unit factor 1 mol K/6.02. ×. 10. 23. atoms K to cancel atoms , which appears in the denominator. The answer is rounded to three digits because the given value and unit factor each have three significant digits. Solution. Calculate the number of moles of potassium iodide in 5.34 ×1025 formula units of KI.
Molecular Mass Worksheet Answer Key. Richard. August 26, 2021. Worksheet Designed To Help Teach Reinforce The Concept Of Determining Percent Composition Of Molecules Teaching Chemistry How To Memorize Things Chemistry Help. Mole Mass And Particle Conversion Worksheet Executive Summary Template Mole Conversion Worksheet Literal Equations.
Worksheet. 1. Molecular mass is measured in _____. atomic mass units. atomic measure units. grams per mole. grams per molecule. 2. The atomic mass of a carbon atom is approximately 12.011 amu.
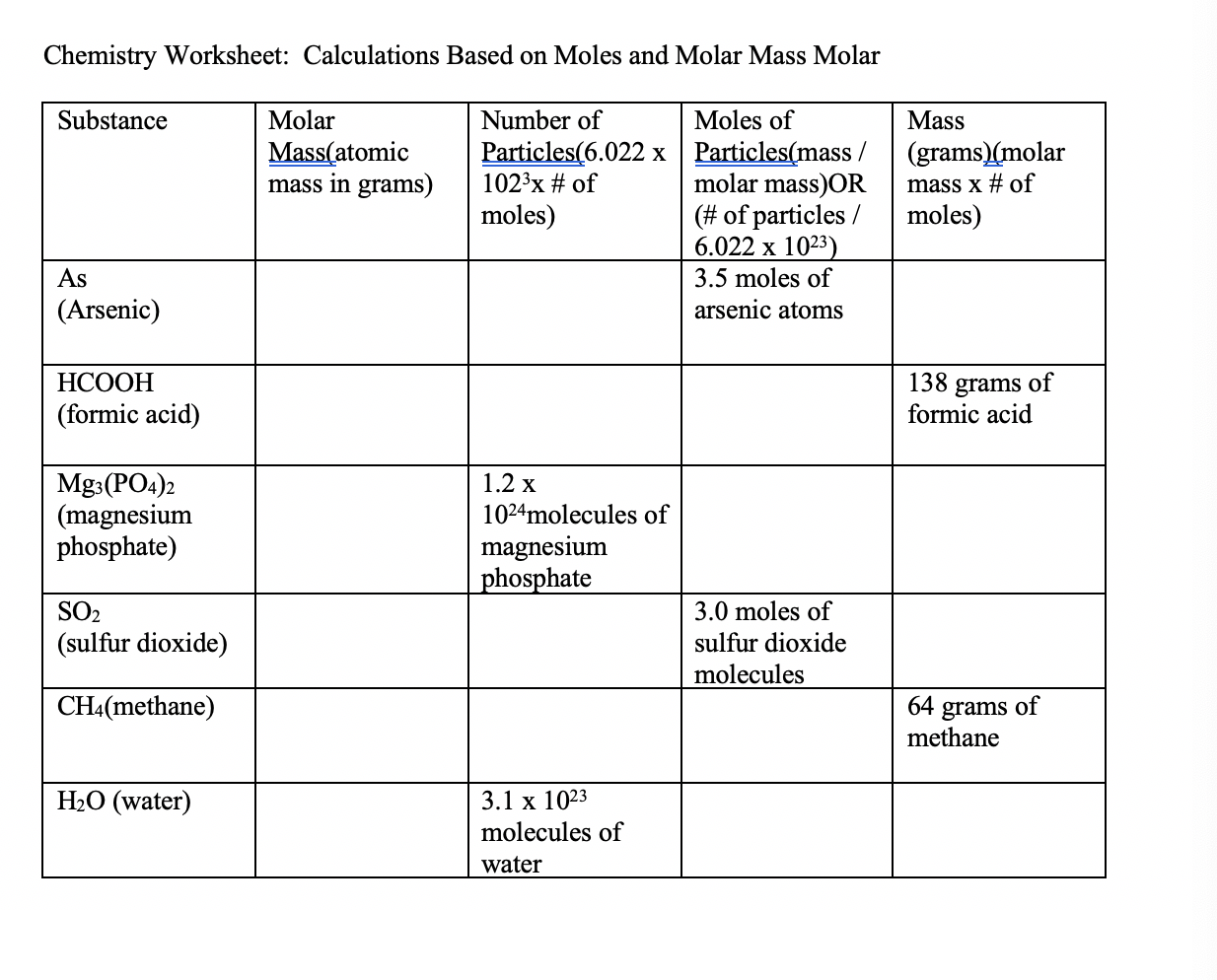
MOLES AND MASS Name Determine the number of moles in each of the quantities below. 2. 3. 4, 25 g of NaCl IV 73.09 125 g of s CIS, 100. g of KMn04kl Ò Cl 35.6) 35 g of CuSO,5H20 Hz q. 6 It 910 gqg Determine the number of grams in each of the quantities below. 2.5 moles of NaCl 2. 3. 4. 0.50 moles of 1.70 moles of KMn04 0.25 moles of KCI Ecl 3.2.
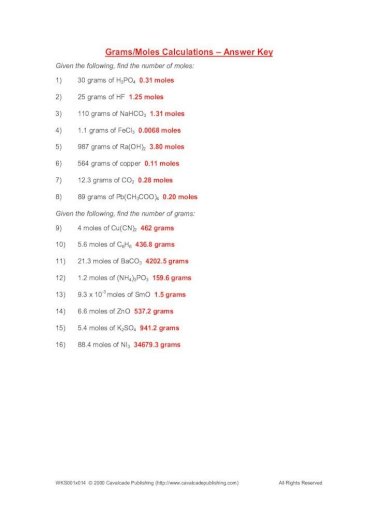
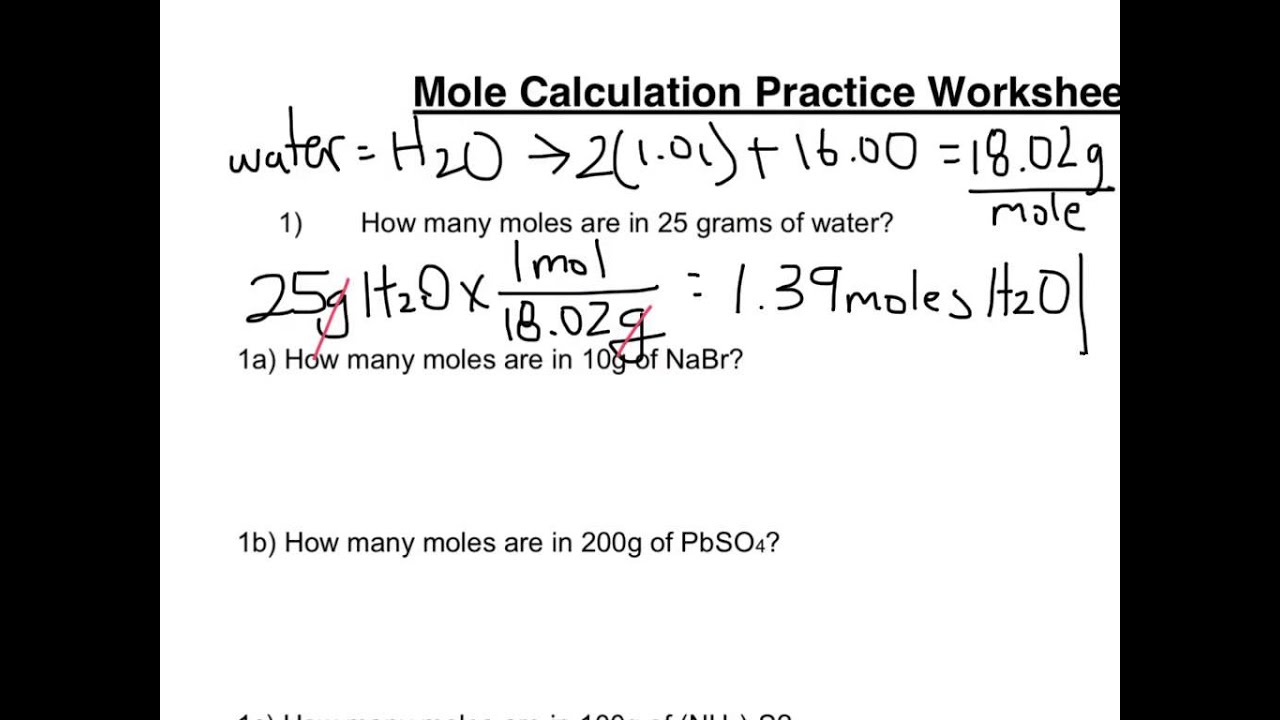
Mole Calculation Worksheet - Answer Key 1) How many moles are in 15 grams of lithium? 0.46 moles 2) How many grams are in 2.4 moles of sulfur? 77.0 grams 3) How many moles are in 22 grams of argon? 0.55 moles 4) How many grams are in 88.1 moles of magnesium? 2141 grams 5) How many moles are in 2.3 grams of phosphorus? 0.074 moles
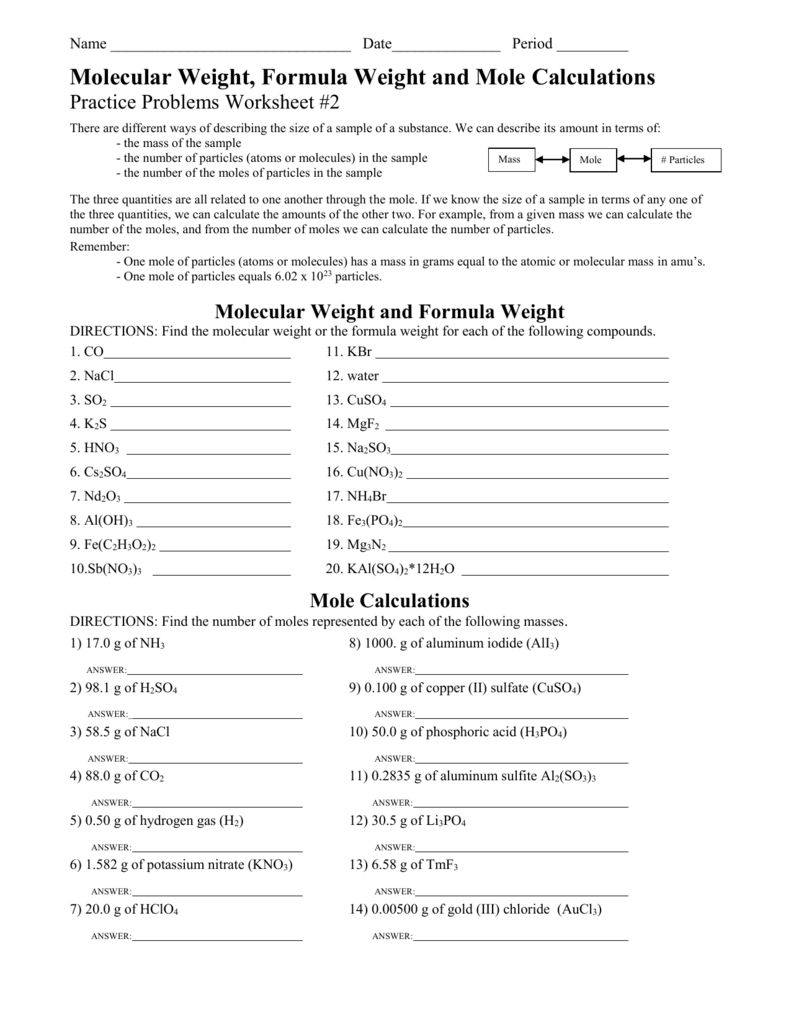
Molar Mass Worksheet W 339 Everett Community College Tutoring Center Student Support Services Program Find the molar masses of the following compounds: 1) LiI 2) PbCO 4 3) Mg(OH) 2 4) K 3 PO 4 5) (NH 4) 3 PO 4 6) C 6 H 12 O 6 7) Fe 2 (SO 4) 3 8) Na 3 P 9) AgF 10) NH 4 C 2 H 3 O 2
Molar mass can be measured by a number of experimental methods, many of which will be introduced in later chapters of this text. Molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. As the name suggests, an empirical formula mass is the sum of the average atomic masses of all the.
Solve the following problems involving the mole concept. (If you are having difficulty go back and review mole worksheets 1-6.) YOU MUST USE COMPLETE AND PROPER SET-UPS. SHOW THE MOLAR MASS CALCULATION WHENEVER THE PROBLEM REQUIRES YOU TO DO ONE. INCLUDE UNITS. CIRCLE YOUR FINAL ANSWER. Problems 1-2: moles to grams AND grams to moles. 1.
Molecular mass and mole calculations. Mole calculation worksheet answer key. 1 mole molar mass could be atomic mass from periodic table or molecular mass 1 mole 224 l of a gas at stp you do not need to worry about this yet each definition can be written as a set of two conversion factors. Molecular Mass And Mole Calculations Worksheet Answers.
Molecular mass and mole calculations. Mole calculation worksheet answer key. 1 mole molar mass could be atomic mass from periodic table or molecular mass 1 mole 224 l of a gas at stp you do not need to worry about this yet each definition can be written as a set of two conversion factors. Mole calculation worksheet answer key.
0.693 moles of this substance has a mass of 55.37 g, determine the identity of element X. 11 An important component of the fast-acting and very effective adhesive Superglue is methyl-2-cyanoacrylate, a compound with the chemical formula C 5 H 5 NO 2. a Determine the molar mass of this compound. b What mass of methyl-2-cyanoacrylate will be.
chemistry worksheet # 1 molar mass (gram molecular/formula weights) We know that grams are actually a measure of the mass of matter and not the weight. Mass is the quantity of matter present; weight is a measure of the pull of gravity on matter and is measured in pounds or newtons.
Mole Calculation Worksheet W 340 Everett Community College Tutoring Center Student Support Services Program 1) How many moles are in 40.0 grams of water?. 2314 mole Cd x 6.022 x 10 atoms Cd = 8.4 x 1023 atoms Cd 1 mole Cd 4) How many moles are in 4.3 x 1022 molecules of H 3 PO 4? 4.3 x 1022 molecules H 3 PO 4
Molecular weight and mole calculations worksheet answers. Compounds - sample questions and two good concept maps Mixtures and Compounds - a short movie featuring iron and sulphur illustrating the difference between a mixture and a compound Jul 04 2020 Relative dating worksheet with answers. 002 moles of NaNO3 3.
Example 1. Computing Molecular Mass for a Covalent Compound Ibuprofen, C 13 H 18 O 2, is a covalent compound and the active ingredient in several popular nonprescription pain medications, such as Advil and Motrin.What is the molecular mass (amu) for this compound? Solution Molecules of this compound are comprised of 13 carbon atoms, 18 hydrogen atoms, and 2 oxygen atoms.
Aug 22, 2021 · The Relative Molecular Mass of a compound is the sum of the masses of all the atoms present in the molecule. B Relative molecular mass of carbon dioxide CO 2 12 216 44 So the molar mass of CO 2 44 g mol-1 Mass of 15 moles of CO 2 number of moles x molar mass 15 x 44 66g.
Displaying top 8 worksheets found for - Molecular Mass And Mole Calculations. Some of the worksheets for this concept are Mole calculations work, Work calculating empirical molecular formulas, Example exercise atomic mass and avogadros number, Calculating empirical and molecular formulas, The mole chemistry lesson plan overview day 11, Chapter 10 chemical alculations and equations, Calculating.
View Answers to Mole Worksheets.pdf from AP PHYSICS 101 at Bryant High School. Molecular Mass and Mole Calculations 7. 29 g 8. 0.0969 mol 9. 3.04 x 10 24 atoms 10. 0.839 mol 11. 148 g 12. 2.769




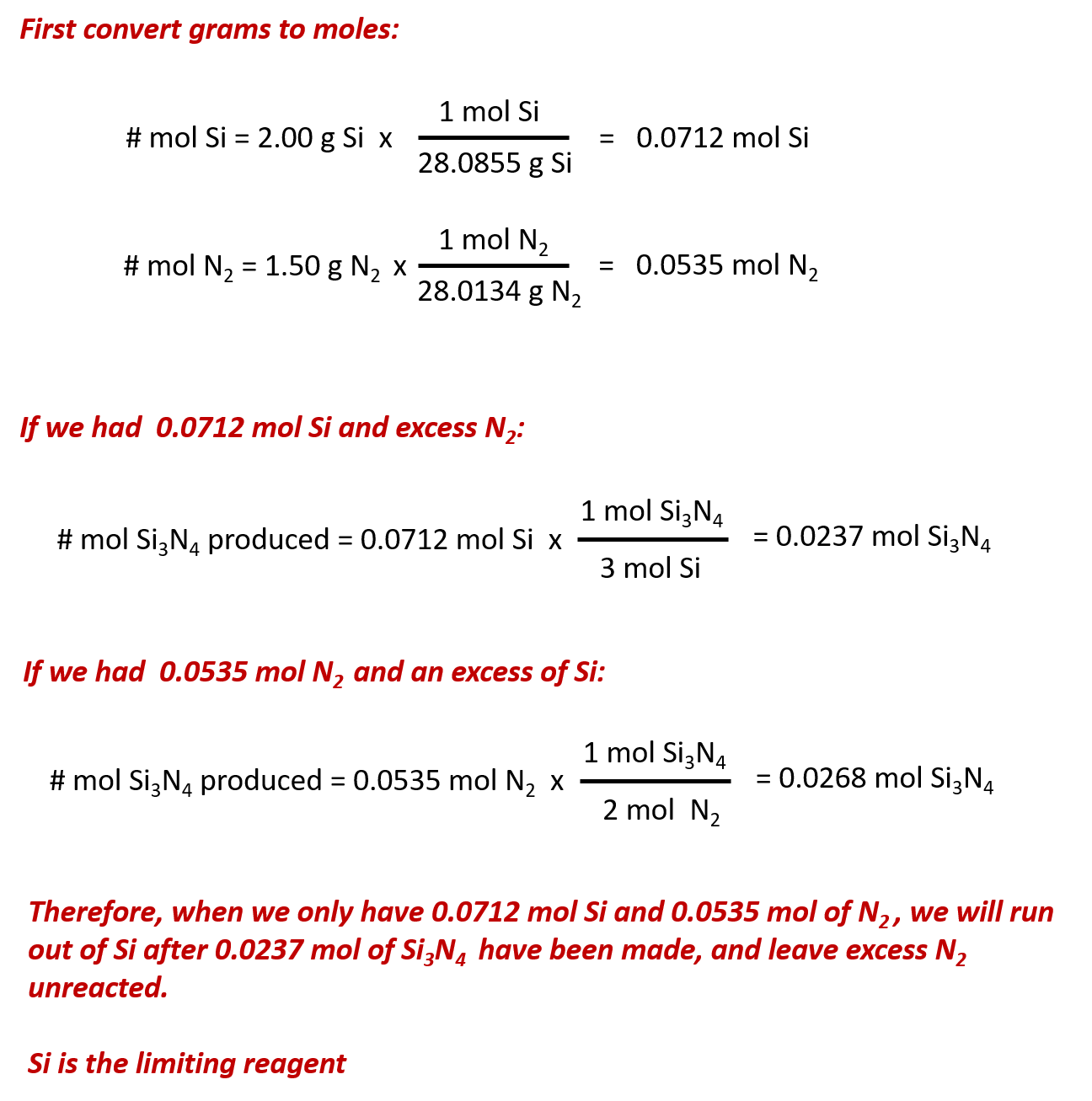

















0 Response to "34 Molecular Mass And Mole Calculations Worksheet Answers"
Post a Comment