32 Chemistry Energy Worksheet Answers
Chemistry Worksheet and Answers Wavelength, frequency, & energy of electromagnetic waves. C = λν E = hν C = 3.00 x 108 m/s h = 6.626 2 x 10-34 J-s (or J/Hz) 1. What is the wavelength of a wave having a frequency of 3.76 x 1014 s-1? 2. Worksheet: Heat and Heat Calculations Name 1. What is the difference in temperature and heat? i s of- how are el ìs an 2. energy is stored is energy in motion. energy and C be measured. can be measured. 3. When you heat a substance and the temperature rises, how much it rises depends upon its mass/ C 0m PO J 4.
About This Quiz & Worksheet. This quiz and worksheet combo helps gauge your knowledge of the characteristics of chemical energy. You will be quizzed on non useable energy and chemical energy.

Chemistry energy worksheet answers
Bond Energy Worksheet. Nitrogen + Hydrogen Ammonia. Use bond energies to determine the energy change for the following reaction: H2(g) + Cl2(g) 2HCl(g) Chemistry*12* Potential*Energy*Diagrams*Worksheet* Name:* Date:* Block:*! USE!THE!POTENTIAL*ENERGY*DIAGRAM!TO!ANSWER!THEQUESTIONS!BELOW:! 1.! Is!the!overall!reaction. Kinetic Energy Practice Problems 1. What is the Kinetic Energy of a 150 kg object that is moving with a speed of 15 m/s? KE = ½ mv2 KE = ? m = 150kg v = 15m/s KE = ½ (150kg) (15 m/s)2 KE = ½ (150kg)(225) KE = 16875J 2. An object has a kinetic energy of 25 J and a mass of 34 kg , how fast is the object moving? KE = ½ mv2 KE = 25J m = 34kg v = ?
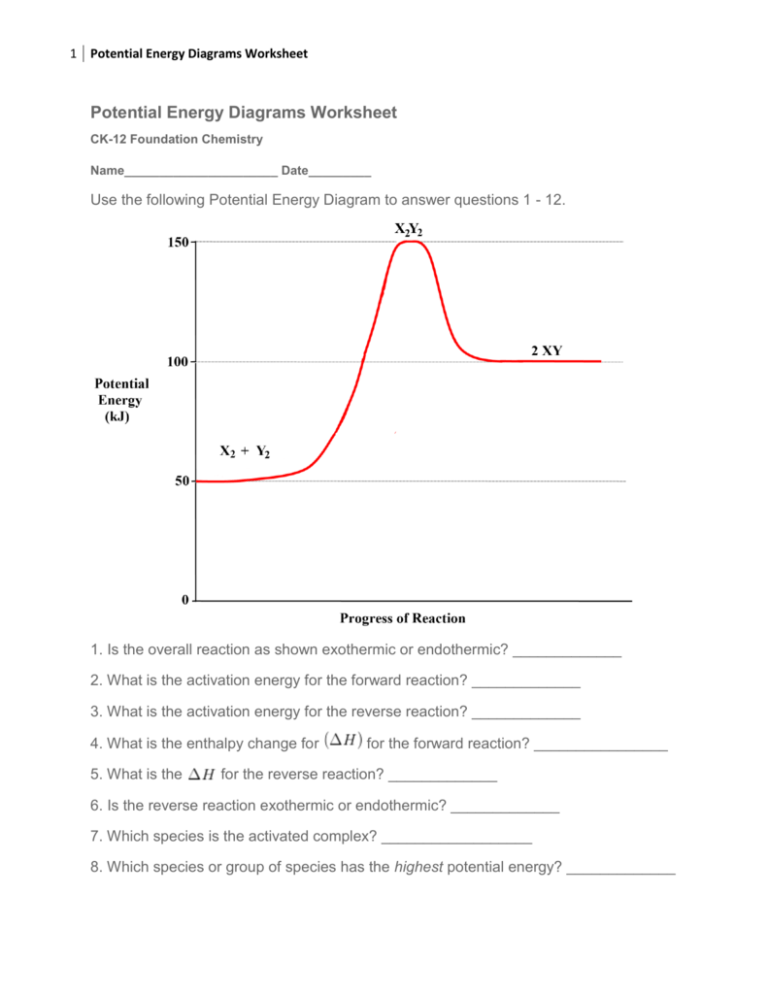
Chemistry energy worksheet answers. Potential Energy Diagrams Worksheet CK-12 Foundation Chemistry Name Use the following Potential Energy Diagram to answer questions 1 - 12. 150 X2Y2 100 Potential Energy (kJ) 50 0 Progress of Reaction 1. Is the overall reaction as shown exothermic or endothermic? 2. What is the activation energy for the forward reaction? Which element in each pair has a larger ionization energy? A number of physical and chemical properties of elements can be predicted from. Use the periodic table and your knowledge of periodic trends to answer the following. See model 1 for specific answers. Periodic table basics worksheet answer key chemistry periodic table, chemistry. Chemistry Worksheet - Wavelength, frequency, & energy of electromagnetic waves. ANSWER KEY Show ALL equations, work, units, and significant figures in performing the following calculations. Identify the type of radiation in each problem. (Use your electromagnetic spectrum) C = λν E = hν C = 3.00 x 108 m/s h = 6.626 2 x 10-34 J-s (or J/Hz) Matter and energy worksheet answer key. Potential energy is energy that is waiting to happen. Which organisms in the food web are producers. Elements are the simplest type of pure substance containing only one type of atom. Which organism is an omnivore. Which organisms would be affected if the producers disappeared. It is stored energy.
High School Chemistry Worksheets and Answer Keys, Study Guides and Vocabulary Sets. CHEMISTRY is the study of matter, its properties, how and why substances combine or separate to form other substances, and how substances interact with energy. The five main branches of chemistry include analytical chemistry, physical chemistry, organic chemistry, inorganic chemistry and biochemistry. Calorimetry Practice Problems (Answers) 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oC? 3135J 3140J (rounded answer for sig. figs.) 2. How many grams of water can be heated from 20.0 oC to 75oC using 12500.0 Joules? 119.6 g 120 g (rounded answer for sig. figs) 3. A physics student skis down a hill accelerating at a constant 20 ms2. Compounds of carbon and oxygen Compound A. A listing of the free printable 8th grade math worksheets available on the site Dilations and scale factors worksheet answer key Chemistry unit 4 worksheet 3 answer key. Unit 3 Worksheet 4 Quantitative Energy Problems - NanoPDF. Indicate how most of the energy is stored in each of the objects. Worksheets are chemistry energy work answer key work chemical energy and atp chemical reactions and energy energy f e kmbt 754 20150622022119 mission 1 what is energy physical and chemical changes work energy forms work. Nh4no2 s n2g 2 h2o g 224 kj b.
Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = temperature Remember, AT = (Tfinal — Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy, and its temperature changes from 25 0 1750C. Energy, Heat, and Work Worksheet - Answer Key. Back to the other Thermodynamics Workbooks and other General Chemistry Workbooks. Go To -> Worksheet - Answer Key - Solutions Manual. What is Energy? The capacity to do work or produce heat. What does the 1 st Law of Thermodynamics State? What else is it known as? Kinetic Energy Practice Problems 1. What is the Kinetic Energy of a 150 kg object that is moving with a speed of 15 m/s? KE = ½ mv2 KE = ? m = 150kg v = 15m/s KE = ½ (150kg) (15 m/s)2 KE = ½ (150kg)(225) KE = 16875J 2. An object has a kinetic energy of 25 J and a mass of 34 kg , how fast is the object moving? KE = ½ mv2 KE = 25J m = 34kg v = ? View Chemistry worksheet - answers.pdf from CHEMISTRY 123 at Simonds High School. MfStrXpg Cs.An RedoxBasics anodeoxidation cathode reduaionFfecth0Ch RIG Card OIL
Chemistry Worksheet - Wavelength, frequency, & energy of electromagnetic waves. ANSWER KEY. Show ALL equations, work, units, and significant figures in performing the following calculations. Identify the type of radiation in each problem. (Use your electromagnetic spectrum)
Temperature is a measure of the average kinetic energy and does not depend upon the amount of matter in the am pl e.H ti sh ok nc rgy fwb u d the amount of matter. K i netcE rgy anot specific heatcapacity Changes in energy heat Potential Energy Raise 1 gram of a substance 1OC.
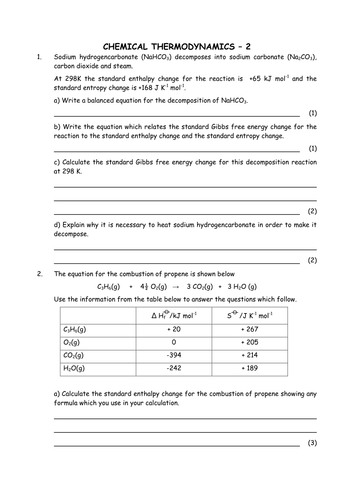
Chemistry: Energy and Stoichiometry Directions: Solve each of the following problems. Show your work, including proper units, to earn full credit. 1. The combustion of propane (C 3 H 8) produces 248 kJ of energy per mole of propane burned. How much heat energy will be released when 1 000 dm3 of propane are burned at STP? 2.
Lattice Energy: Definition, Trends & Equation. Worksheet. 1. A reaction's heat of formation is: The amount of energy needed to add an electron to an atom or ion. The total change in energy when a.
Chemistry*12* Potential*Energy*Diagrams*Worksheet* Name:* Date:* Block:*! USE!THE!POTENTIAL*ENERGY*DIAGRAM!TO!ANSWER!THEQUESTIONS!BELOW:! 1.! Is!the!overall!reaction.
Chemistry Worksheet - Wavelength, frequency, & energy of electromagnetic waves. ANSWER KEY Show ALL equations, work, units, and significant figures in performing the following calculations. Identify the type of radiation in each problem. (Use your electromagnetic spectrum) C = λν E = hν C = 3.00 x 108 m/s h = 6.626 2 x 10-34 J-s (or J/Hz)
Bond Energy Chem Worksheet 16 2 Answers 1/5 Kindle File Format Bond Energy Chem Worksheet 16 2 Answers Chemistry Atoms First 2e-Paul Flowers 2019-02-14 Chemistry for the Biosciences-Jonathan Crowe 2010 Focuses on the key chemical concepts which students of the biosciences need to understand, making the scope of the book directly relevant to the.
Chemistry Worksheet - Wavelength, frequency, & energy of electromagnetic waves. ANSWER KEY Show ALL equations, work, units, and significant figures in performing the following calculations. Identify the type of radiation in each problem. (Use your electromagnetic spectrum) C = λν E = hν C = 3.00 x 108 m/s h = 6.626 2 x 10-34 J-s (or J/Hz)
Chemistry 12 Worksheet 1-2 - Potential Energy Diagrams TO THE PROGRESS OF RERCTION the overall reaction as shown exothermic or endothermic' 2 What is the the reaction? 30 is Of (AH) + so 50 ICU Page I 6. IS reaction or 7, Which species S. WNch or of energy?
Bond Energy Worksheet. Nitrogen + Hydrogen Ammonia. Use bond energies to determine the energy change for the following reaction: H2(g) + Cl2(g) 2HCl(g)
Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other.
Department of Chemistry University of Texas at Austin 4.The question above describes the photoelectric effect. Use the space below to draw a picture illustrating this effect. Describe this figure and explain how frequency and work function (Φ) relate to the kinetic energy of the emitted electron. 1 photon ! 1 !!
Introduction to energy worksheet answer key. A race care traveling at its maximum speed c both forms. Determine the best match between basic types of energy and the description provided. The two basic types of energy. A skier at the top of the mountain a kinetic energy 2. A skier at the top of the mountain a kinetic energy b 2.
Castle Learning Matter Review Answer Key- Given out in class 9/29/16. 1) 2 Elements can NEVER be decomposed chemically. 2) 4 Solids have a regular geometric pattern. 3) 2 Density is an intensive property that is the same for any sample size of a substance. 4) 3 Gases have indefinite shape and indefinite volume. 5) 4.
Q=mc(T 2-T 1) or Q=mc∆T. 6. A block of brass is heated from 22oC to 78oC. The mass of the block is 15 kg. How much thermal energy must be added to the block of brass? list known values formula substitution answer & units
CHEMISTRY 85 80 75 70 65 60 55 Temp. (QC) 5 0 40 35 30 25 20 15 10 10 -15 -20 Date. CHEMISTRY HEATING CURVE WORKSHEET 8. In what part(s) of the curve would increasing kinetic energy be displayed?... What is the mass of the coaster if it has 19 ,08ðJ of potential energy at point 3? (answer is in kg) 7001 28m '15m
In the process, the substance absorbed 569 calories of energy. What is the specific heat of the substance? What is the specific heat of an unknown substance if a 2.50 g sample releases 12 calories as its temperature changes from 25°C to 20°C? ANSWER KEY. HEAT Practice Problems. Q = m x ∆T x C . 5.0 g of copper was heated from 20°C to 80°C.
Answer: a) Lattice energy b) Electron affinity c) Heat of formation 3. a. Draw Born-Haber cycle for the formation of calcium oxide. b. Use the following data to calculate the lattice energy of calcium oxide. You must write all thermochemical equations for the steps of the cycle. The enthalpy of formation of calcium oxide (solid) = - 636 kj/mole
Student Worksheet for Thermochemistry Attempt to work the following practice problems after working through the sample problems in the videos. Answers are given on the last page(s). Relevant Equations/Information Specific Heat: Q=mc∆T ∆T= Final Temperature - Initial Temperature






















0 Response to "32 Chemistry Energy Worksheet Answers"
Post a Comment