35 Limiting Reagent And Percent Yield Worksheet
Limiting Reagents and Percentage Yield Review Worksheet 1. Consider the reaction I 2 O 5 (g) + 5 CO(g) -----> 5 CO 2 (g) + I 2 (g) a) 80.0 grams of iodine(V) oxide, I 2 O 5, reacts with 28.0 grams of carbon monoxide, CO. Determine the mass of iodine I 2, which could be produced? b) If, in the above situation, only 0.160 moles, of iodine, I 2 5) If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? Limiting Reagent Worksheet All of the questions on this worksheet involve the following reaction: When copper (II) chloride reacts with sodium nitrate, copper (II) nitrate and sodium chloride are formed.
Created Date: 1/27/2016 7:41:57 AM

Limiting reagent and percent yield worksheet
Transcribed image text: Folsom Lake College - Chemistry 305 Stoichiometry, Percent Yield, Limiting Reagent Worksheet Stoichiometric Calculations from Balanced Equations Your Name: Date: The coefficients in a balanced chemical equation represent the numbers of moles of reactants and products. Using a balanced chemical equation, one can make quantitative predictions about the chemicals involved. What is the percent yield for the reaction? N 2 (g) + 3 H 2 (g) 2 NH 3 (g) • 16.0 g is the ACTUAL YIELD (given) 28.3 g is the THEORETICAL YIELD (calculated) • Now that you found out the theoretical value, plug your answer into the formula percent yield = 16.0 g × 100 = 56.7 % 28.3 g x 100 theoretical yield actual yield percent yield = The limiting reagent is the reagent that determines the quantity of product that may be formed using a response. Often, it’s essential to recognize the limiting reagent in an issue. Limiting Reactant and Percent Yield Worksheet Answer Key with Percent Yield Worksheet 1 Kidz Activities.
Limiting reagent and percent yield worksheet. What is the percent yield for the reaction? N 2 (g) + 3 H 2 (g) 2 NH 3 (g) • 16.0 g is the ACTUAL YIELD (given) 28.3 g is the THEORETICAL YIELD (calculated) • Now that you found out the theoretical value, plug your answer into the formula percent yield = 16.0 g × 100 = 56.7 % 28.3 g x 100 theoretical yield actual yield percent yield = ii) what percentage yield of iodine was produced. 2. Zinc and sulfur react to form zinc sulphide according to the equation. Zn + S -----> ZnS If 25.0 g of zinc and 30.0 g of sulfur are mixed, a) Which chemical is the limiting reactant? Transcribed image text: Folsom Lake College - Chemistry 305 Stoichiometry, Percent Yield, Limiting Reagent Worksheet Stoichiometric Calculations from Balanced Equations Your Name: Date: The coefficients in a balanced chemical equation represent the numbers of moles of reactants and products. Using a balanced chemical equation, one can make quantitative predictions about the chemicals involved. Calculate the theoretical yield and the percent yield. Cu + Cl2 ( CuCl2. 8) In the reaction of Zn with HCl, 140.15 g of ZnCl2 was actually formed, although the theoretical yield was 143 g. What was the percent yield? Zn + HCl ( ZnCl2 Limiting Reagent Worksheet -KEY
Limiting Reactant and Percent Yield Practice Name_____ 1) Consider the following reaction: NH 4 NO 3 + Na 3 PO 4 (NH 4) 3 PO 4 + NaNO 3 Which reactant is limiting, assuming we started with 30.0 grams of ammonium nitrate and 50.0 grams of sodium phosphate. What is the mass of each product that can be formed? What is the percent yield? Write balanced reaction Cu + S Cu2S 2 Determine theoretical yield – doing a mass to mass problem 1.50g Cu 1 mol Cu 1mol Cu2S 159.17g Cu2S 63.55g Cu 2 mol Cu 1mol Cu2S = 1.88 g Cu2S Percent Yield = 1.76 g x 100 = 93.6 % 1.88g Limiting Reagent Worksheet #1 1. Given the following reaction: (Balance the equation first!) C 3H 8 + O 2-----> CO 2 + H 2O a) If you start with 14.8 g of C 3H 8 and 3.44 g of O 2, determine the limiting reagent b) determine the number of moles of carbon dioxide produced c) determine the number of grams of H 2O produced The limiting reagent is the reagent that determines the quantity of product that may be formed using a response. Often, it’s essential to recognize the limiting reagent in an issue. Limiting Reactant and Percent Yield Worksheet Answer Key with Percent Yield Worksheet 1 Kidz Activities.











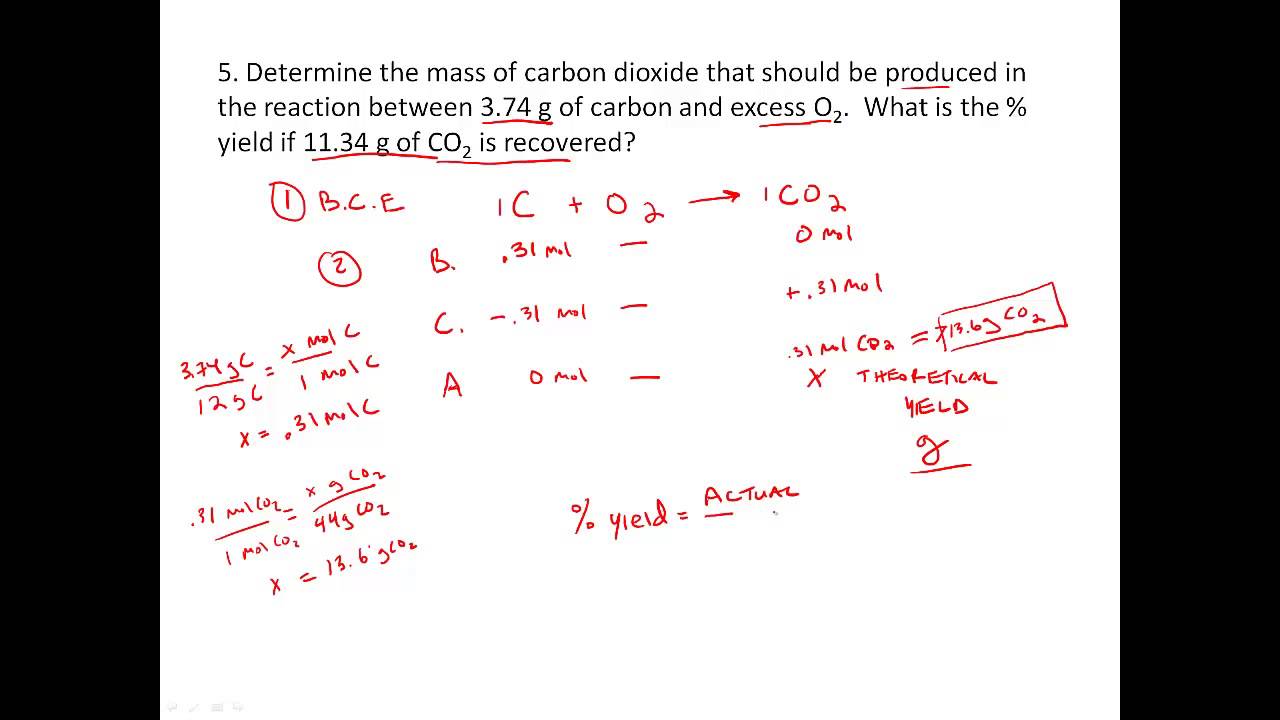












0 Response to "35 Limiting Reagent And Percent Yield Worksheet"
Post a Comment