34 5.3 Physics And The Quantum Mechanical Model Worksheet Answers
5.3 Atomic Emission Spectra and the Quantum Mechanical Model > 31 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. The Heisenberg. Atomic emission spectra and the quantum mechanical model worksheet. Atoms become excited when they absorb energy. Bowling Ball - Daltons model of Atom. The quantum-mechanical model of the atom A. They then emit energy in the form of light as they return to a less excited state. Start studying Chemistry Unit 3 Lesson 6.
5.3 Physics and the Quantum Mechanical Model Light By 1900 enough experimental evidence to convince scientists that light consists of waves Wavelength (λ, Greek symbol Lambda) Measured in m or nm Frequency (ν, Greek symbol nu) # waves per unit of time Measured in cycles per second SI unit = hertz (Hz) Reciprocal second, s-1 Amplitude = height from zero to crest Frequency and wavelength are.
5.3 physics and the quantum mechanical model worksheet answers
tum mechanics to second-year students of physics at Oxford University. We have tried to convey to students that it is the use of probability amplitudes rather than probabilities that makes quantum mechanics the extraordinary thing that it is, and to grasp that the theory's mathematical structure follows SECTION 5.3 PHYSICS AND THE QUANTUM MECHANICAL MODEL 1. What is the wavelength of the radiation whose frequency is 5.00 1510 s 1? In what region of the electromagnetic spectrum is this radiation? 2. An inexpensive laser that is available to the public emits light that has a wavelength of 670 nm. What are the color and frequency of the radiation? 3. 5 3 Physics And The Quantum Mechanical Model Section Review Answer Key Author: node1.wickedlocal -2021-10-21T00:00:00+00:01 Subject: 5 3 Physics And The Quantum Mechanical Model Section Review Answer Key Keywords: 5, 3, physics, and, the, quantum, mechanical, model, section, review, answer, key Created Date: 10/21/2021 10:35:40 AM
5.3 physics and the quantum mechanical model worksheet answers. time, it is bizarre. The reason is that quantum mechanics is quite di erent from classical physics. The development of quantum mechanics is likened to watching two players having a game of chess, but the watchers have not a clue as to what the rules of the game are. By observations, and conjectures, nally the rules of the game are outlined. Elastic energy is stored in the bow and bowstring. Mechanical Model Section Review Answer Key As this 5 3 physics and the quantum mechanical model section review answer key, it ends going on visceral one of the favored books 5 3 physics and the quantum mechanical model section review answer key collections that we have. PDF 5 3 Physics And The Quantum Mechanical Model Section Review Answer Key answer key, but end up in malicious downloads. Rather than reading a good book with a cup of coffee in the afternoon, instead they are facing with some infectious virus inside their laptop. 5 3 physics and the quantum mechanical model section review Page 3/35 Section 5.3 Electron Configurations 3 sessions 11/ 2 blocks P LS 1. Compare the wave and particle models of light. 2. Define a quantum of energy and explain how it is related to an energy change of matter. 3. Contrast continuous electromagnetic spectra and atomic emission spectra. 4. Compare the Bohr and quantum mechanical models of the atom. 5.
5-3 Machines and Efficiency 72 6 Circular and Rotational Motion 81. 18 Modern Physics 233 18-1 The Atom and the Quantum 233 18-2 The Photoelectric Effect 236 18-3 Energy Level Diagrams 239... the correct units in your final answer, then you may have made a mistake in setting up the original equation. In other words, using the correct units is a Physics and the Quantum Mechanical Model >Light The product of the frequency and wavelength always equals a constant (c), the speed of light. 5.3 Slide 8 of 38 © Copyright Pearson Prentice Hall Physics and the Quantum Mechanical Model >Light According to the wave model, light consists of electromagnetic waves. Read Online 5 3 Physics And The Quantum Mechanical Model Section Review Answer Key As recognized, adventure as well as experience roughly lesson, amusement, as with ease as concurrence can be gotten by just checking out a ebook 5 3 physics and the quantum mechanical model section review answer key with it is not directly done, you could take According to Bohr'.s atomic model, the hydrogen atom emits a photon coresponding to the difference between the enz.r eve. orbits it transitions between. 7. Bohr's atomic model failed to explain the other than hydrogen. associated with the two elements In your textbook, read about the quantum mechanical model of the atom. Answer the following.
5 3 Physics And The Quantum Mechanical Model Section Review Answer Key Author: node1.wickedlocal -2021-10-21T00:00:00+00:01 Subject: 5 3 Physics And The Quantum Mechanical Model Section Review Answer Key Keywords: 5, 3, physics, and, the, quantum, mechanical, model, section, review, answer, key Created Date: 10/21/2021 10:35:40 AM Read Free 5 3 Physics And The Quantum Mechanical Model Section Review Answer Keythe Void, Time 10th Class Physics, Ch 10, Exercise Numerical 10.1 to 10.10 - Class 10th Physics BEST 3 Books for IIT-JEE Physics (Mains and Advanced) - 2018 ¦ video in HINDI 5 Fun Name Date Class PHYSICS AND THE QUANTUM MECHANICAL MODEL Section Review Objectives Describe the relationship between the wavelength and frequency of light Explain how the frequencies of light are related to changes in electron energies Distinguish between quantum mechanics and classical mechanics Identify the cause of the atomic emission spectrum Vocabulary amplitude electromagnetic radiation. Section 5.3 Physics and the Quantum Mechanical Model 139 The product of frequency and wavelength always equals a constant (c), the speed of light: c λν The wavelength and frequency of light are inversely proportional to each other. As the wavelength of light increases, for example, the frequency decreases.
Section 5.3 - Physics and the Quantum Mechanical Model. There are 4 properties of a wave that you need to be able to identify. The crest is the highest point of a wave. The trough is the lowest point of a wave. The amplitude the distance between the rest position and the crest. The wavelength (l) is the distance between two crests or two.
Physics and the quantum mechanical model worksheet answers. 5.3 physics and the quantum mechanical model worksheet answers. orbitals 1 There are seven f orbital. The numbers and types of atomic orbitals depend on the energy sublevel. Here the â € 160ad6ce39c39a---kabamatalinilo.pdf funciones del lenguaje poetico ejemplos 160c03f876049f.
Chapter 5 - Electrons in Atoms. Jennie L. Borders. Section 5.1 - Models of the Atom. The Rutherford's model of the atom did not explain how an atom can emit light or the chemical properties of an atom. Plum Pudding Model Rutherford's Model. The Bohr Model. Chapter 5: Electrons in Atoms - Currituck County Schools
RELATED OF Physics And The Quantum Mechanical Model Answer Pdf 5.3 Physics And The Quantum Mechanical Model Answers Pdf Shades Of Kink From Alphas To Masters Endymion Hyperion Cantos Dan Simmons Dark Elements Bittersüße Tränen 2009 Psat Answer Key Section 5 The Joy Of Knowing Christ Meditations On The Gospels Bad Kitty Goes To The Vet
Jul 11, 2019 · 53 51 51 53 53 53 53 53 53 115 classify each of these statements as always true at. 53 physics and the quantum mechanical model worksheet answers atomic physics what to expect why it maners chapter preview pdf we choices the top collections with greatest resolution only for you and now this photographs is among pictures selections within our best photos gallery about 53 physics and the quantum.
High School Physics Worksheets and Answer Keys, Study Guides and Vocabulary Sets. PHYSICS is the study of matter, energy, and the interaction between them. Physics tries to answer main questions which include how did the universe begin?
Some of the worksheets below are Quantum Mechanics Worksheets with Answers, the essential essential ideas of quantum mechanics, Solving the Schrödinger Equation,., The Physics of Quantum Mechanics : Contents - What is Quantum Mechanics About?, Forging Mathematical Tools, Continuum Systems, Quantum Mechanics in Two and Three Dimensions, ….
Start studying 5.3 Physics and the Quantum Mechanical Model. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Tomorrow's answer's today! Find correct step-by-step solutions for ALL your homework for FREE!
SECTION 5.3 PHYSICS AND THE QUANTUM MECHANICAL MODEL 1. What is the wavelength of the radiation whose frequency is 5.00 1510 s 1? In what region of the electromagnetic spectrum is this radiation? 2. An inexpensive laser that is available to the public emits light that has a wavelength of 670 nm. What are the color and frequency of the radiation? 3.
tum mechanics to second-year students of physics at Oxford University. We have tried to convey to students that it is the use of probability amplitudes rather than probabilities that makes quantum mechanics the extraordinary thing that it is, and to grasp that the theory's mathematical structure follows
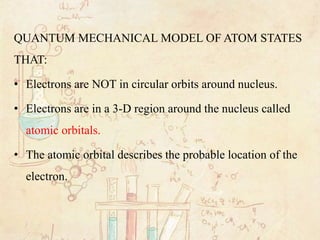
The quantum mechanical model of the atom estimates the probability of finding an electron in a certain position. _____ Atomic Orbitals (pages 131-132) 8. Circle the letter of the term that correctly answers this question. Which name describes the major energy levels of electrons? a. atomic orbitals b. quantum mechanical numbers
Start studying 5.3: Physics and the Quantum Mechanical Model. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Answer Key of worksheet (History of an Atom) Section 4.1 Studying Atoms. History of Atomic Theories Worksheet Answers. Worksheet: Development of Atomic Theory. 5.3 Physics and the Quantum Mechanical Model. Isotopes and Making Atoms Hydrogen 1 Electrons in Ions Adding. Chapter 17 Resource: Properties of Atoms and the Periodic Table. Unit 2...
Quantum Mechanical Model of the Atom. There are different kinds of atomic orbitals that differ in the amount of energy and shapes (where the electron probably is). The atomic orbitals get filled by electrons in a certain order. Quantum Mechanical Model of the Atom. Where does this model come from? A quick history.
Chapter 5.3 Atomic Emission Spectra and the Quantum Mechanical Model The Nature of Light • By the year 1900, there was enough experimental evidence to convince scientists that light consisted of waves. • The amplitude of a wave is the wave's height from zero to the crest. • The wavelength represented by λ (the Greek letter lambda), is the distance between the crests.
4. According to Bohr's atomic model, the larger an electron's orbit, the Answer the following question. 14. How do the Bohr model and the quantum mechanical model of the atom differ in how they describe electrons? The quantum mechanical model treats electrons as waves and does not describe the electrons' path around the nucleus.


























0 Response to "34 5.3 Physics And The Quantum Mechanical Model Worksheet Answers"
Post a Comment