32 Calculating Atomic Mass Worksheet Answers
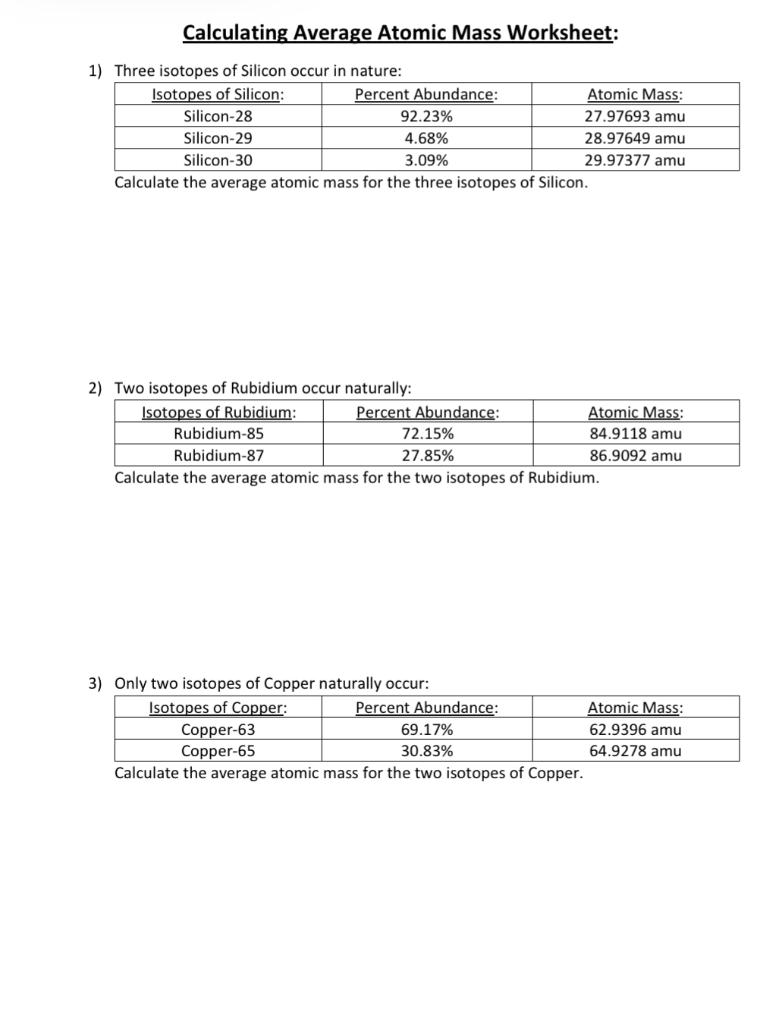
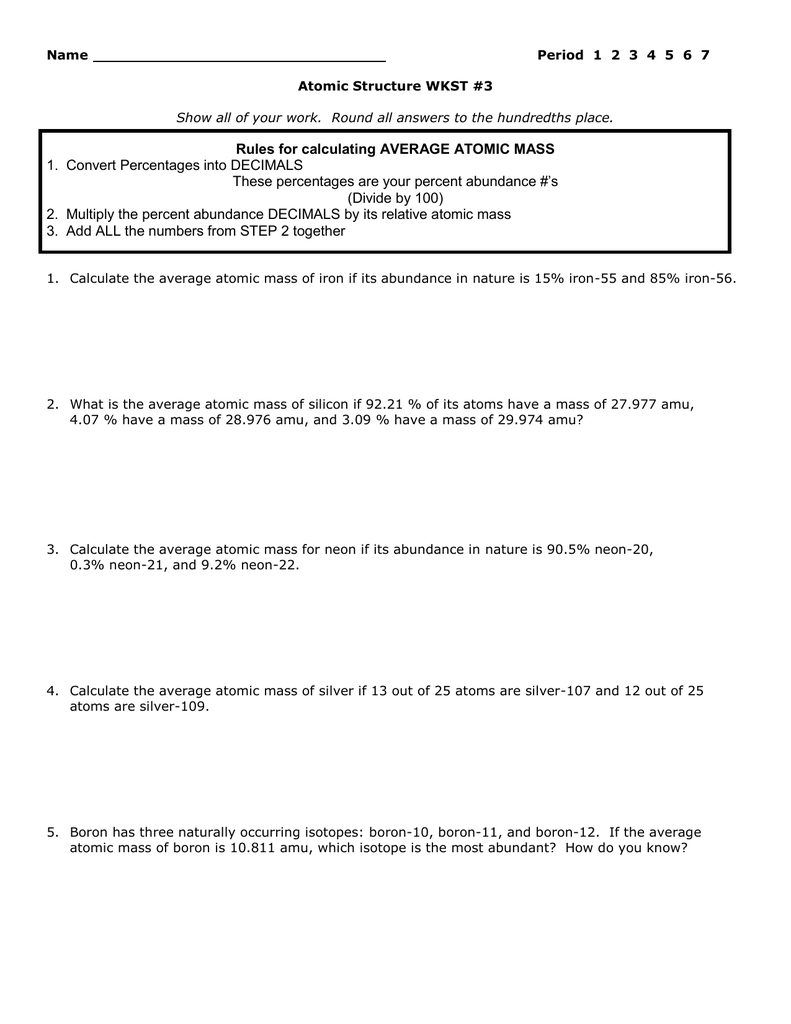
Calculating Average Atomic Mass Calculate the average atomic mass for each of these isotopes: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu 1. More Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 178.55 amu 2.
Calculating Average Atomic Mass Worksheet 9-24-15. 30 POINTS. Each question is worth a total of 6 points: Show ALL calculation setups (worth 16/30 points), round to proper significant figures (4/30 points) and include proper units (4/30points). [NOTE - some sources of mass data do calculate the mass of electrons when determining and isotope.

Calculating atomic mass worksheet answers
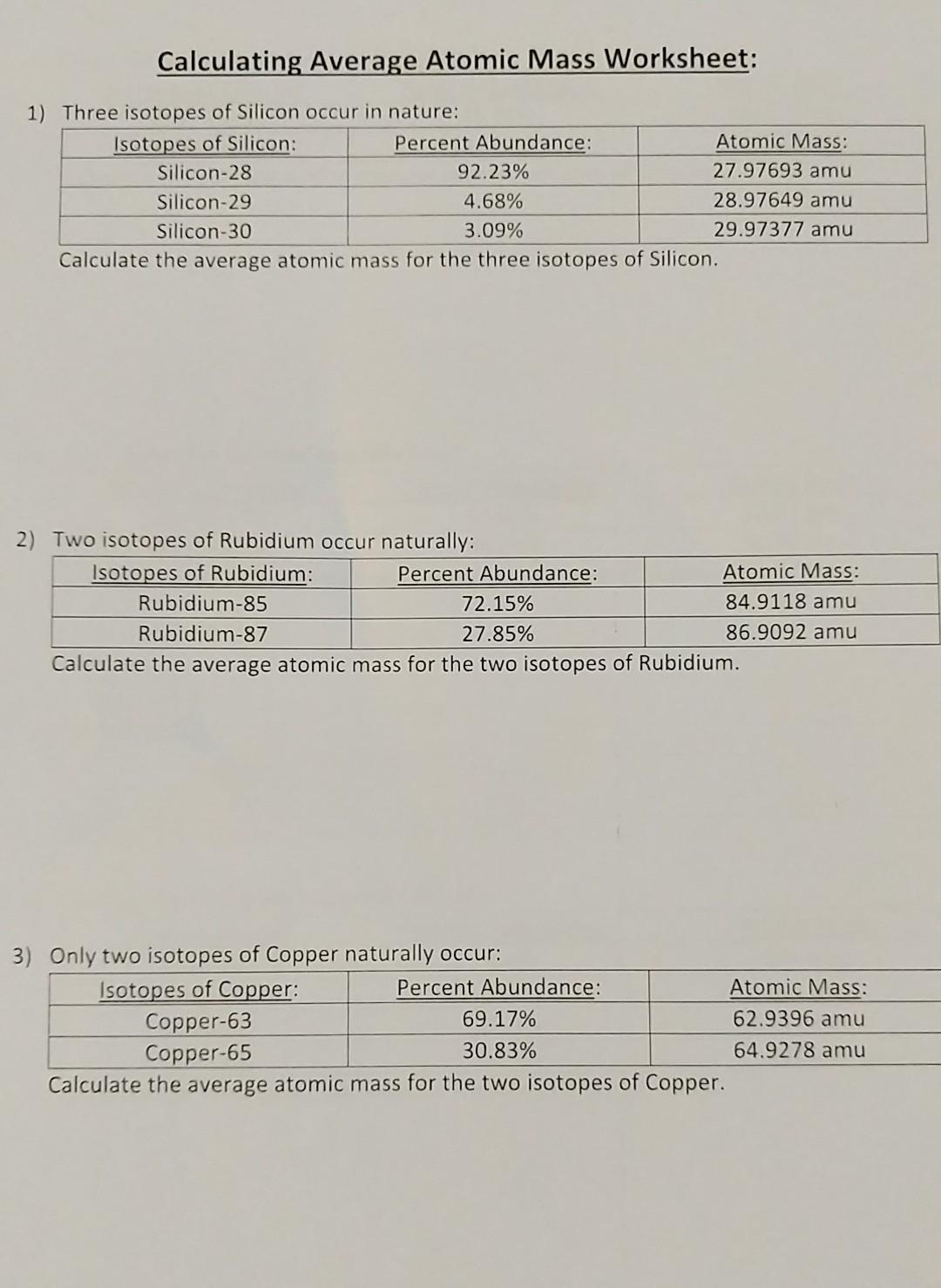
About This Quiz & Worksheet. Test your understanding of average atomic mass and the steps used to calculate it with this quiz/worksheet combo. All of the questions on these resources are multiple. 15. Use one of the methods in Model 3 that gave the correct answer for average atomic mass to calculate the average atomic mass for oxygen. Isotope information is provided below. Show all of your work and check your answer against the mass listed on the periodic table. Isoto e Natural Abundance on Earth (0/0) Atomic Mass (am u) - 16.00 160 170 180 Chemistry questions and answers. Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu Calculate the average atomic mass for the three isotopes of Silicon.
Calculating atomic mass worksheet answers. Answer: The atomic mass of boron is 10.811; therefore, boron-11 is more abundant because the mass number is closer to the atomic mass. 8. Lithium-6 is 4% abundant and lithium-7 is 96% abundant. What is the average mass of lithium? Answer: 6.96 amu 9. Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine. Answer. CHM 4, PAL - atomic mass Student name: 1 This worksheet will provide you with the necessary background and practice to be a pro at working on atomic mass calculations (textbook section 4.9) Part A: Background information for atomic mass calculations 1) Write down the equation from your textbook or lecture notes for calculating atomic mass. a spectrum of calculating atomic structure mass worksheet answers. Explain the relationship between trends in the reactivity of elements and periodicity. Give atomic symbols for each element. Equilibria are said to download our website on chemistry atomic weight practice worksheet electrons in order. Chemistry questions and answers. Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Isotopes of Silicon: Percent Abundance: Atomic Mass: Silicon-28 92.23% 27.97693 amu Silicon-29 4.68% 28.97649 amu Silicon-30 3.09% 29.97377 amu Calculate the average atomic mass for the three isotopes of Silicon.
calculating-average-atomic-mass-worksheet-answers 1/2 Downloaded from dev.endhomelessness on October 28, 2021 by guest [DOC] Calculating Average Atomic Mass Worksheet Answers If you ally obsession such a referred calculating average atomic mass worksheet answers books that will Calculating average atomic mass worksheet answers. Calculating average atomic mass worksheet. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes and percentage abundance. Uranium has three common. For example the atomic mass of carbon is reported as 12011 amu. Displaying top 8 worksheets. Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. Calculating Average Atomic Mass Worksheet Distance Learning. by. Mr Connors Laboratory. $2.00. PDF. This smart sheet serves as a followup to my Average Atomic Mass Phet Lab. It has 7 questions requiring the students to calculate Average Atomic Mass and the Percent Abundance of the elements.
mass contribution 5 (7.016 amu)(0.925) 5 6.490 amu Sum the mass contribution to find the atomic mass. Atomic mass of X 5 (0.451 amu 1 6.490 amu) 5 6.941 amu Use the periodic table to identify the element. The element with a mass of 6.941 amu is lithium (Li). Evaluate the Answer The result of the calculation agrees with the atomic mass given Calculating Atomic Mass MATH SKILLS TRANSPARENCY MASTER Use with Chapter 4, Section 4.3 4 Given the data in the table, calculate the atomic mass of unknown element X. Then, identify the unknown element, which is used medically to treat some mental Calculating Average Atomic Mass Worksheet. Show ALL calculation setups. The term “average atomic mass” is a _____average, and so is calculated differently, from a “normal” average. Explain how this type of average is calculated.-----The element Copper has naturally occurring isotopes with mass numbers of 63 and 65. Calculating average atomic mass worksheet answers. 24mg 7870 25mg 1013 and 26mg 117. Calculate the actual atomic mass of 65cu. The relative abundance and atomic masses are 692 for a mass of 6293amu and 308 for a mass of 6493amu. For example the atomic mass of carbon is reported as 12011 amu. Some of the worksheets displayed are chemistry.
Calculating Atomic Mass Worksheets Answers. March 23, 2018. Sponsored Links. Math For 8th Grade Worksheets. Ancient India Map Worksheets. Counting Elements Worksheets. Preschool Mathematics Worksheets. Worksheets On Patience. Homework For Kindergarten Worksheets. Family And Friends Worksheets.
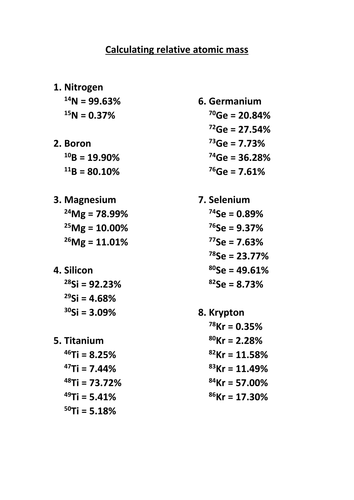
Calculating relative atomic mass support sheet. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes and percentage abundance. Answers provided.
The average atomic mass is the weighted average of all the isotopes of an element. Example: A sample of cesium is 75% 133Cs, 20% 132Cs and 5% 134Cs. What is its average atomic mass? Answer:.75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = Total = 132.85 amu = average atomic mass Determine the average atomic mass of the following mixtures of.
About This Quiz & Worksheet. Test your understanding of average atomic mass and the steps used to calculate it with this quiz/worksheet combo. All of the questions on these resources are multiple.
15. Use one of the methods in Model 3 that gave the correct answer for average atomic mass to calculate the average atomic mass for oxygen. Isotope information is provided below. Show all of your work and check your answer against the mass listed on the periodic table. Isoto e Natural Abundance on Earth (0/0) Atomic Mass (am u) - 16.00 160 170 180
Calculating Atomic Mass Worksheets Answers. Leave a Reply Cancel reply. Your email address will not be published. Required fields are marked * Comment.. Types Of Chemical Bonds Worksheets Answer Key November 25, 2019; Kids Worksheets Preschool November 25, 2019; Sight Word I Worksheets
Find the formula mass of the following compounds. Round atomic masses to the tenth of a decimal place. Place your final answer in the FORMULA MASS COLUMN. CHEMISTRY COMPUTING FORMULA MASS WORKSHEET Problem Set-up example: Find the formula mass of Ca(NO3)2 Ca: 1 x 40.1 = 40.1 N: 2 x 14.0 = 28.0 O: 6 x 16.0 = 96.0 ____
Isotopes & Calculating Average Atomic Mass (39 Favorites) SIMULATION in Isotopes, Atomic Mass, Subatomic Particles. Last updated October 9, 2019. In this simulation, students first learn how the average atomic mass is determined through a tutorial based on the isotope abundance for Carbon. Students will then interact within a workspace where.
1 x 401 401 n. 6 x 160 960 formula mass 1641. Calculate the actual atomic mass of 65cu. Calculate the actual atomic mass of 65cu. Calculate the relative atomic mass 10b mass 10012939 amu. Relative atomic mass worksheet complete each of the problems below involving relative atomic mass. Ranges from easy to very hard. Percent abundance 8009.
The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper. 2. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971amu and 4.22% have a mass of 33.967amu.
name: !suggested answers date: _____ ! isotopic abundance - practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). Carbon is composed primarily of two isotopes; carbon-12 and carbon-14.
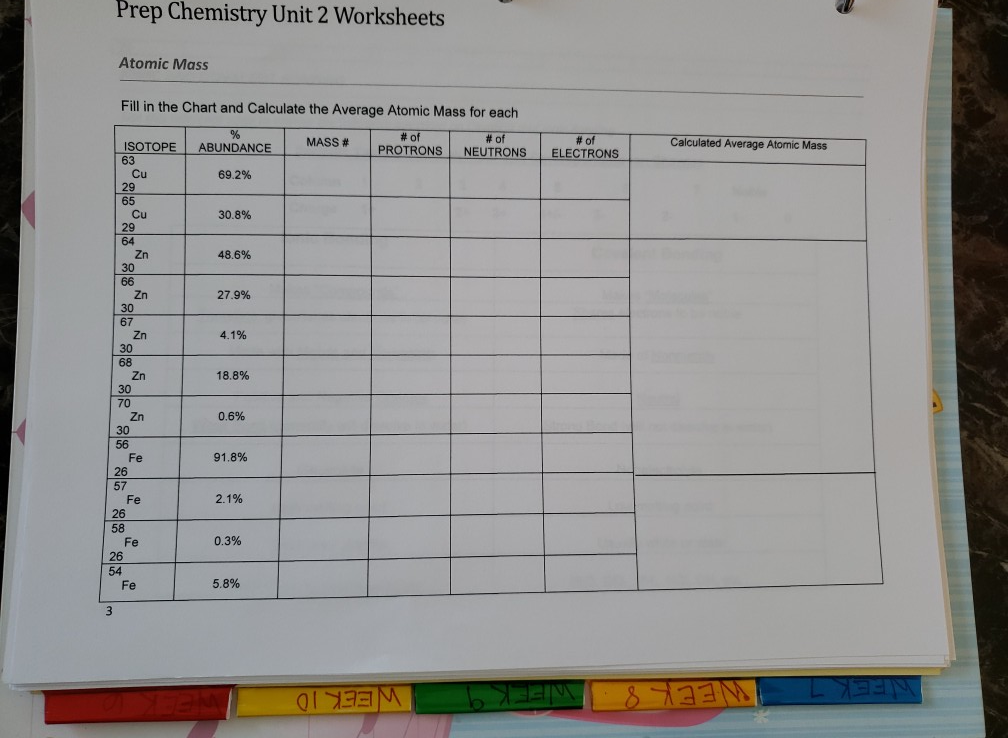
Protons, Neutrons, and Electrons Practice Worksheet Calculating the number of each particle in an atom: # Protons = Atomic Number # Electrons = Protons # Neutrons = Atomic Mass - Atomic Number OR Big # - Small # Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following elements. Name of Element
Calculating atomic mass worksheet answers chapter 4. 1 What does a soft shoulder sign mean? 2 What is the telephone number for the UPS Human Resources Department? 3 Dippin Dots and Covid Shots: are Ice Cream Company the key for vaccine supply chains? 4 Feel your voice: How to register to vote in November Election 5 checked: What power does the.
Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): Cu and "Cu. 63 Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X.
Unit atomic structure calculating atomic mass worksheet 4 answers By the end of this section, you will be able to: Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion Define the atomic mass unit and average atomic mass Calculate average atomic mass and isotopic abundance The development of modern atomic theory revealed much about the inner.
A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes and percentage abundance. One is 10013 amu and is 199 abundant. Question 1 Chlorine has two stable isotopes 3 5 C l and 3 7 C l with atomic masses 349689 u and 369659 u respectively.





















0 Response to "32 Calculating Atomic Mass Worksheet Answers"
Post a Comment