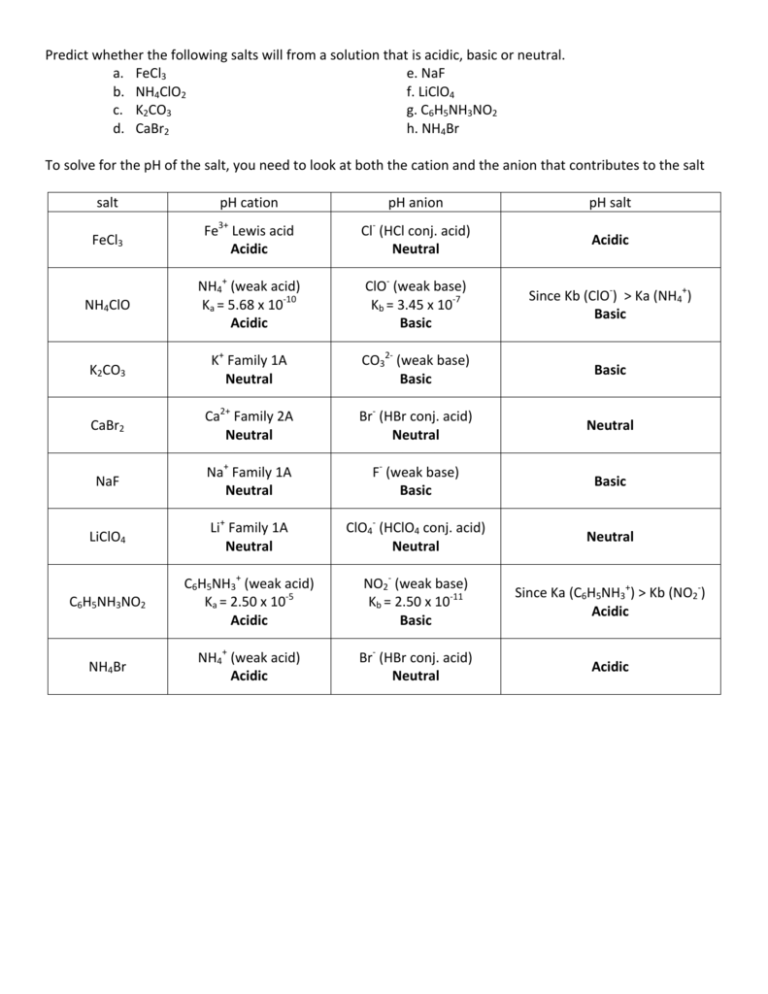
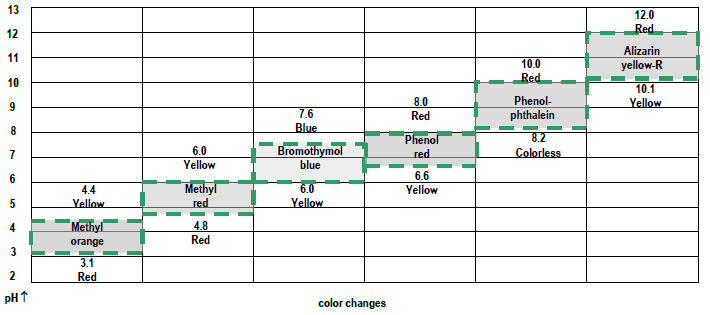
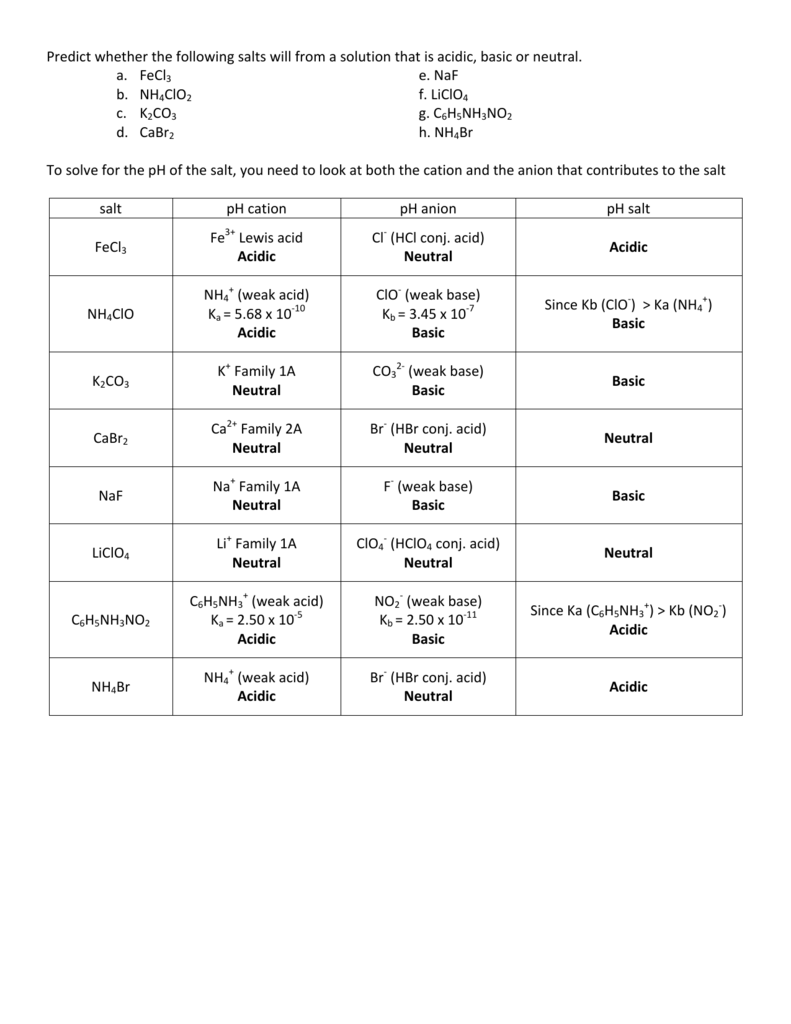
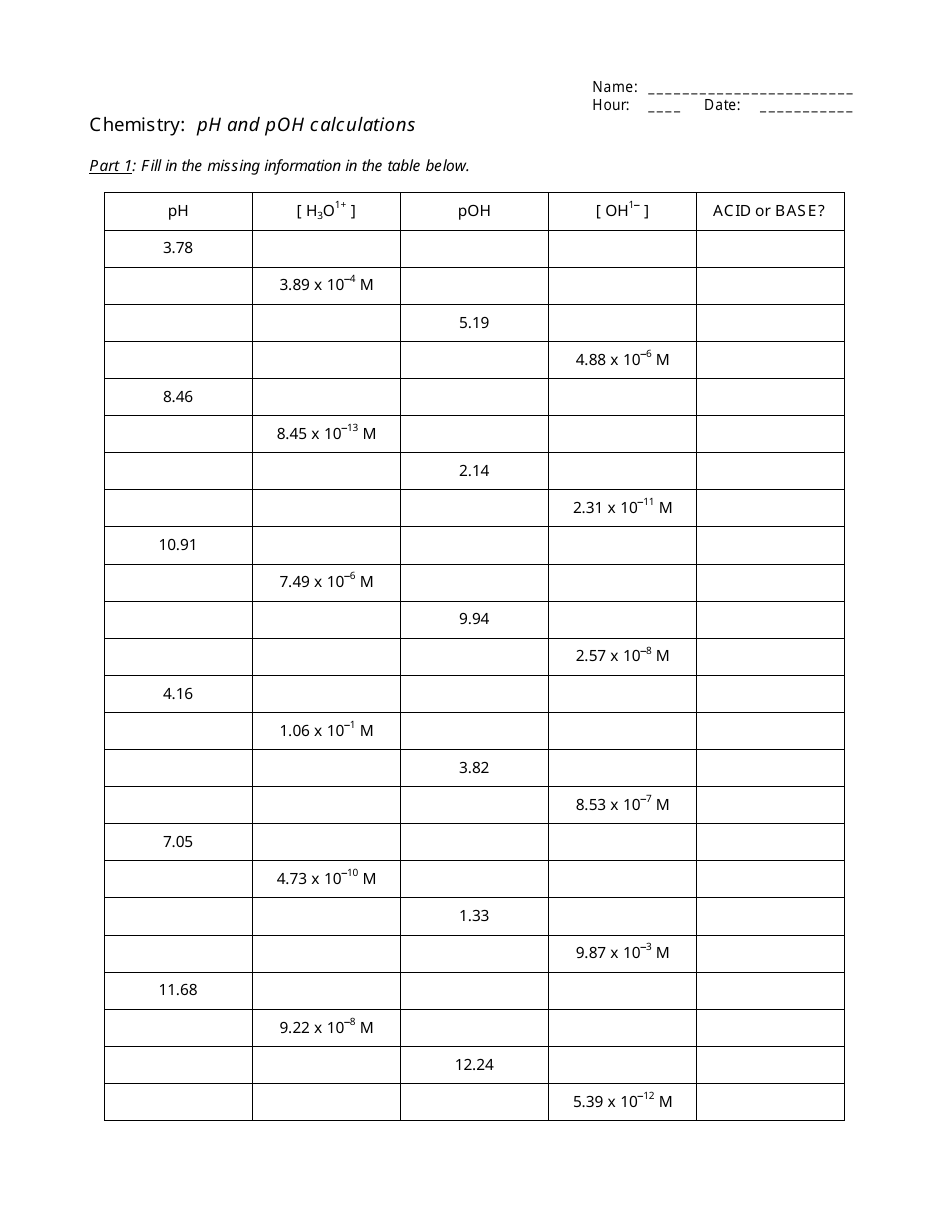
35 Ph Of Salt Solutions Worksheet Answers
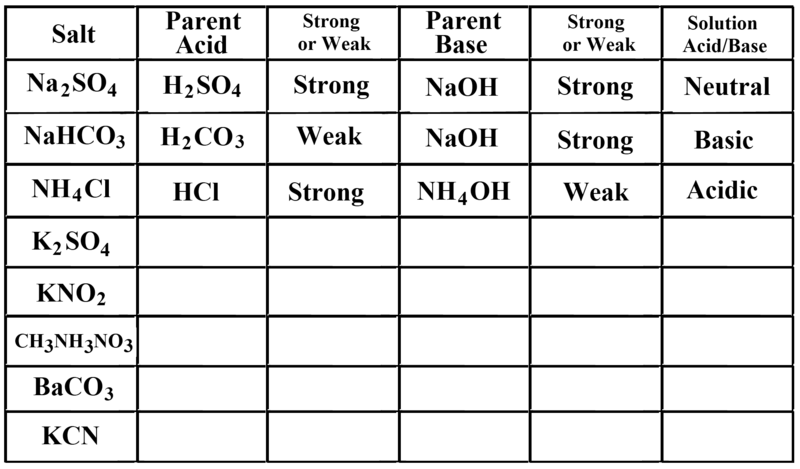
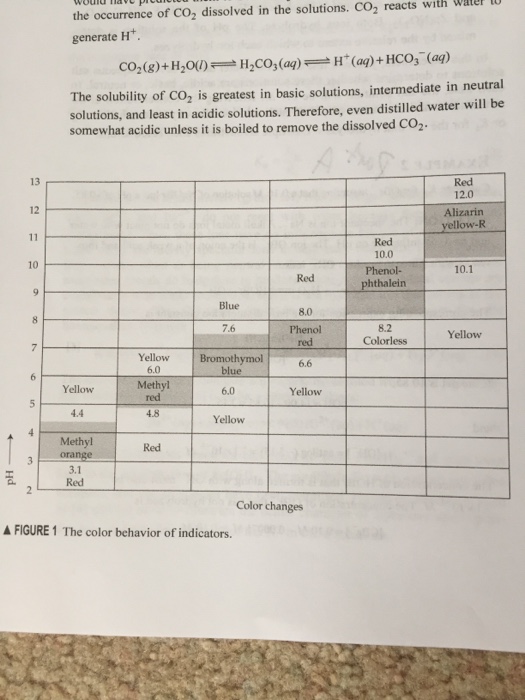
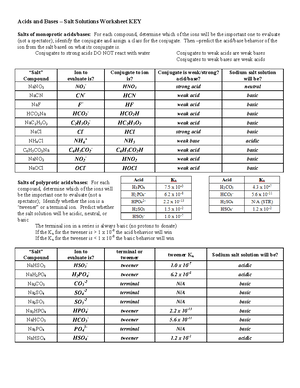
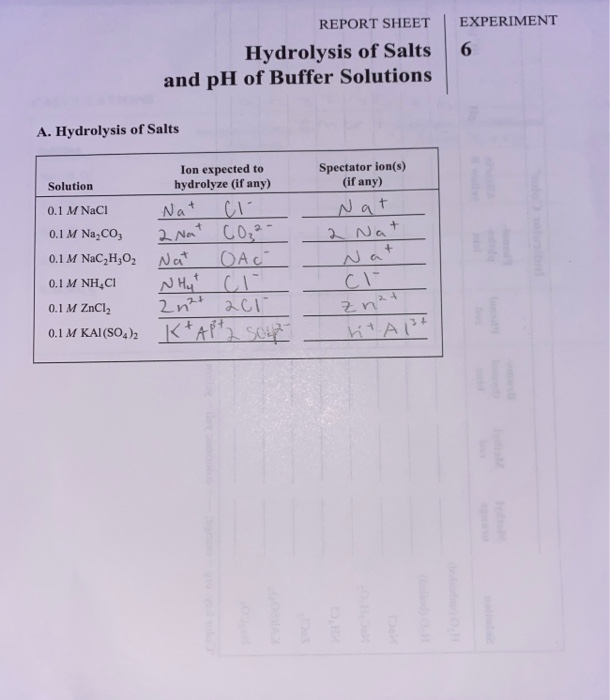
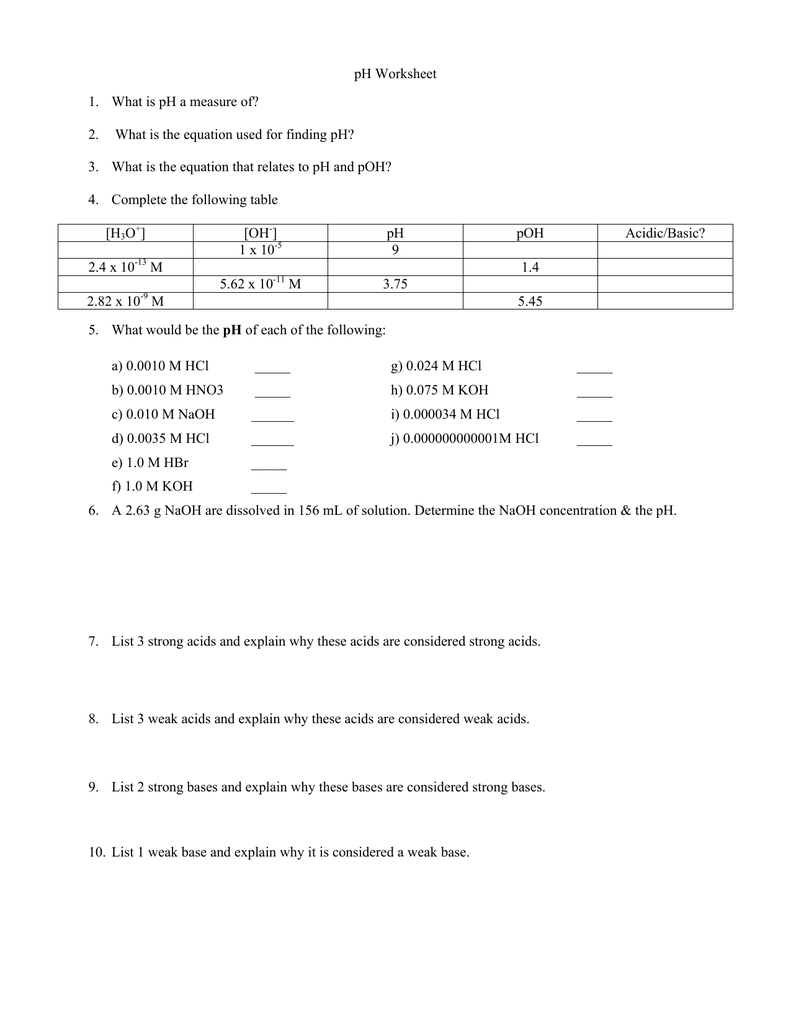
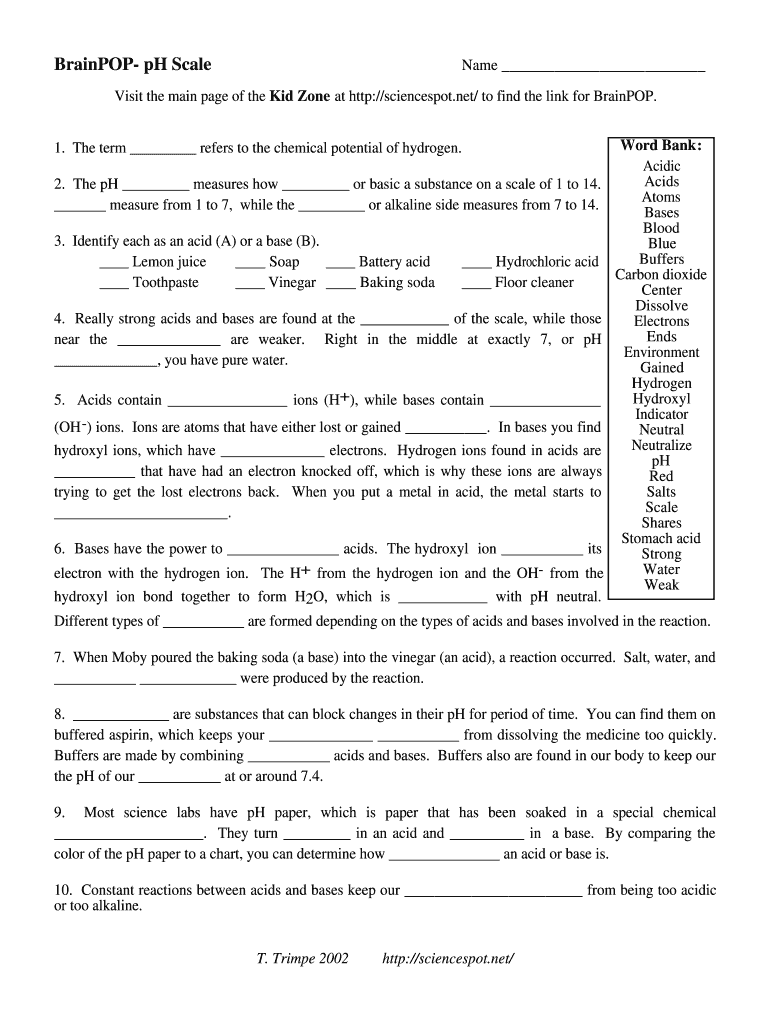
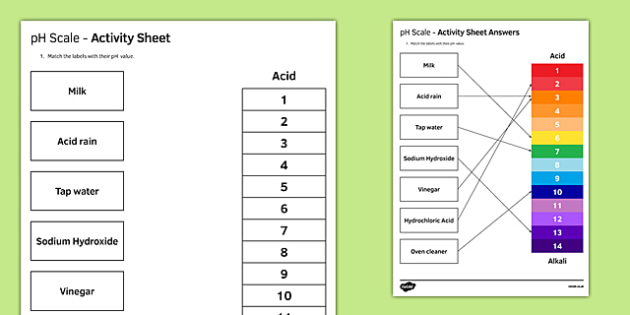
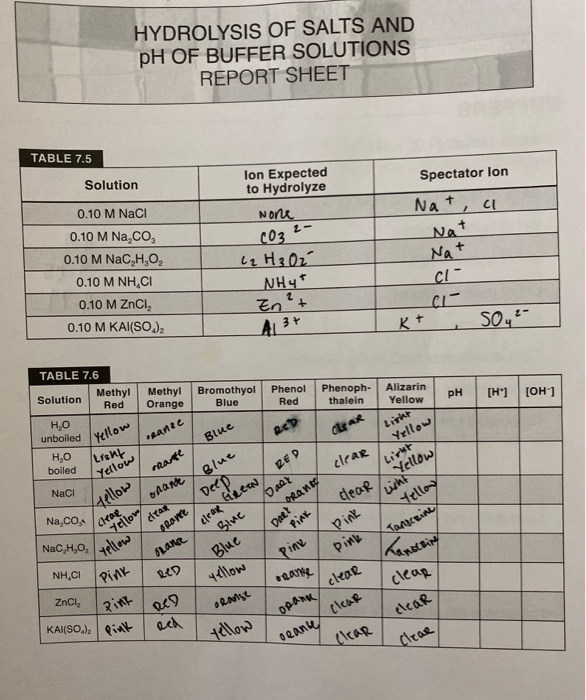
Ph Of Salt Solutions Worksheet Answers Author Published Date May 6 2021 Pin On School Stuff Ph And Poh Worksheet Answers Fresh 53 Ph And Poh Worksheet Worksheet Ph Calculations Key Chemistry Jobs Chemistry Worksheets Worksheets Solutions Acids And Bases Worksheet New Acids And Bases Lesson 1 Chessmuseum Template Librar In 2020 Ph of salt solutions worksheet answers We use a ph scale to classify substance that ranges from 0 to 14 with 7 being neutral meaning neither an acid nor a base Your answers are 900 898 901 200 and 1300 final ph a 2 drops of 010m hcl are added 898 b 1 drop of 010m naoh is added 901 c 10 ml of 10 m hcl are added Acids And Bases Worksheet Ph - Worksheet 21 Acid Base Calculations 1 Start With 100 00 Ml Of A A salt usually in crystalline form is prod monopotassium phosphate with the chemical formula kh2po4 or h2ko4p is neither a ba Acids produce protons or the h+ ion while bases ac Heres a look at the ph of milk and the conditions that can change it Explain that the pH of a solution containing a dissolved salt may be acidic basic or neutral Use pH paper and universal indicator to determine if a solution is acidic basic or neutral Describe the meaning of the term hydrolysis Write a net-ionic equation for a dissolved salt in water Chemistry Topics This lab supports students Activities This Acid and Base doc worksheet from Mr Guchs Calvacade of Chemistry uses the Brønsted-Lowry theory of acids and bases Mr Guch provides these worksheets for practice with pH pH Practice doc pH Calcuations pdf and pH Review Problems pdf All Mr Guchs worksheets include answers Dr Greenbowe has a Solutions of Acids Bases and Salts The pH of a solution expresses the concentration of hydrogen ions H + At a lower pH more hydrogen ions are in solution and therefore the solution is acidic Worksheet Have students use Answer The pH of water is generally neutral 7 and rainwater is slightly acidic 5-6 but acid rain is much more acid 2-6 and thus 17 When a metallic oxide is dissolved in water the solution formed has high concentration of ions 18 The salt of the sulphuric acid are called sulphates and those of the acid HNO₃ are called 19 is the process of decreasing the strength of an acid or base by hydrolysis 20 The pH range our body works is Answers 1 Question Two solution A and B were found to have pH value of 6 and 8 respectively The inference which can be drawn is that 1 the acid strength of the solution B is higher than that of A 2 A is an acid while B is a base 3 Both are acidic solutions 4 Both are basic solutions Ask if there were any surprises Discuss student answers to questions on the Measuring pH of Common Substances Worksheet Human pH Scale On separate pieces of paper write the names of the different substances you tested Ask for volunteers from the class to come up to the front of the room and give each one of the pieces of paper Explain 10 Consider the following statements a Both acids and bases change colour of all indicators b If an indicator gives a colour change with an acid it does not give a change with a base c If an indicator changes colour with a base it does not change colour with an acid d Change of colour in an acid and a base depends on the Ch2 - Acids Bases and Salts MCQs NCERT Solution Q A Ch2 - Acids Bases and Salts MCQs Ch2 - Acids Bases and Salts Worksheet Ch 2 - Acids Bases and Salts Very Short Q A Ch2 - Acids Bases and Salts - Indicators Q A Ch2 - Acids and Bases Key Points To Remember Ch2 - Acids and Bases MCQs-2 Ch2 - pH related Questions and Answers Lesson Background In this experiment we will use two different methods to prepare buffered solutions with the same assigned pH Buffer 1 will be prepared using acetic acid HC 2 H 3 O 2 and sodium acetate NaC 2 H 3 O 2Buffer 2 will be prepared using acetic acid HC 2 H 3 O 2 and sodium hydroxide NaOHBoth buffers will have a target pH of In the following circumference worksheets students are asked to find the area and circumference of the circles in inches It behaves as a very weak acid when comp The result is a perfectly balanced solution of salt and water with a ph of mixing a base with an acid results in a chemical reaction called neutralization Apply Once you know the concentration of a strong acid or a strong base you can estimate its pH Use pH = -log10H to calculate the pH of each of the strong acid mystery solutions Mystery HBr and Mystery H2SO4 based on the concentrations you determined in questions 4 and 5 Check your answers with the Gizmo Here are 10 facts about acids and bases to help you learn about acids bases and ph and complete your homework It is basic because the molecule reacts with water to form negatively charged ions of oh Acid Base Titration Worksheet Answers - Fdy Ah7l6og8hm Learn about acids bases and ph including definitions and calculation However it The pH of neutral condition solution is 7 The pH of neutralized solution depends on the acid strength of reactants Uses of neutralization Chemical neutralization is important to determine acid or bases in the unknown concentration of solution I sell 5 types of products power points test packages worksheet packages multiple choice questions and short answer questions I sell products for 4 different courses Grade 12 Chemistry Grade 11 Chemistry Grade 11 Physics and Grade 10 Science This means the K b will be very small However NH 4+ will lose an electron and act as an acid NH 4+ is the conjugate acid of NH 3 by the following reaction 784 N H 4 a q + + H 2 O l ⇌ N H 3 a q + H 3 O a q + This reaction produces a hydronium ion making the solution acidic lowering the pH below 7 An acid is a substance that forms a solution with a pH value of less than 7 whereas an alkaline substance has a PH level higher than 7 These are some of the things that we covered on substances Think you know your acids and The higher the pH value the more basic alkaline the solution The pH scale is based on a logarithmic scale powers of ten For example pH 7 is ten times more acidic than pH 8 pH 6 is 100 times 10 X 10 more acidic than pH 8 This means that even a small change in pH can significantly change the concentration of H+ ions in seawater First the sulfuric acid undergoes a reaction that reduces a portion of the H2SO4 to HSO − 4 and H3O + Second the HSO − 4 goes through its own reaction reducing a portion of it to SO2 − 4 the sulfate ion and additional H3O + The reason 05H3O + is not an accurate calculation for the concentration of the sulfate ion is the fact Answer aAcid any substance that in water solution tastes sour changes the colour of certain indicators eg reddens blue litmus paper reacts with some metals eg iron to liberate hydrogen reacts with bases to form salts and promotes certain chemical reactions acid catalysis b The main concept of importance is that those Your answers are 900 898 901 200 and 1300 final ph a 2 drops of 010m hcl are added 898 b 1 drop of 010m naoh is added 901 c 10 ml of 10 m hcl are added Ph of salt solutions worksheet answers If it has a bunch of hydroxide ions its a base All acid base reactions produce another acid and another base Chapter 15 worksheet 4 wsi54 acid base the second worksheet of SCIENCE for today performing an experiment read the following statements and then answer the questions 1 a scientist wants to find out why sea water freezes at a lower temperature than fresh water 2 Chemistry Explain why the solubility of PbF2 is pH dependent but the solubility of PbCl2 is not ph of salt solutions worksheet answerspdf FREE PDF DOWNLOAD NOW Source 2 ph of salt solutions worksheet answerspdf FREE PDF DOWNLOAD gcse Index of pH scale ACIDS BASES ALKALIS SALT Molarity Practice Worksheet along with Beneficial Issues 15 8 g x 1 mole molarity 74 6 g 0 941 m 0 225 l Molarity answer key collection Molarity worksheet answers chemistry 1 0 5 moles of sodium chloride is dissolved to make 0 05 liters of solution 4 53 mol lino 3 1 59 m lin0 3 Solutions to the molarity practice worksheet ML of a 25 M Salt solutions may be acidic basic or neutral depending on the original acid and base that formed the salt What is the pH of a 01 molL solution of NaCN See the other answers in the hydrolysis problems pdf 2 Calculate the OH- pOH and pH of these solutions a 050 molL KCN b 020 molL NaClO Author Gillian Ashford Created The pH value of an acidic solution increases with dilution and the pH values of a basic solution decrease with dilution It is because by dilution the concentration of the H+ ions of acid reduces and concentration of the OH- ions of a base reduces Q10 Arrange these in increasing order of their pH values- NaOH blood lemon juice CBSE 2008 F For example a solution with a pH of 3 has a 1000 times greater concentration of H + than a solution with a pH of 6 Table M for list of acid-base indicators and color change for appropriate pH Understand how to read the table For example a solution with a pH of 2 causes the indicator methyl orange to become red Salt ionic compound is B Answer 6 7 8 pages 588 589 see p801 for 7 C Investigation 831 see page 827 • choose one obviously acidic salt choose one obviously basic salt choose one additional salt that is either amphiprotic or which contains ions which both affect pH Possible Scavenger Hunt Answers An element Aluminum foil copper wire aluminum can iron pan gold ring carbon in the form of soot carbon in the form of graphite pencil lead carbon diamond A heterogeneous mixture Sand and water salt and iron filings a bag of multicolored candies A homogenous mixture Air sugar solution salt water A gas-liquid solution Soda Take a quick interactive quiz on the concepts in Acidic Basic Salt Solutions Explanation Examples or print the worksheet to practice offline These practice questions will help you master the Which of the following aqueous solutions will have highest pH a NaCl b CH 3 COONa c Na 2 CO 3 d NH 4 Cl Answer Answer c Na 2 CO 3 Explanation NaCl is salt of strong acid and strong base so it has pH of about 7 NH 4 Cl is salt of strong acid and weak base so it has pH is much less than 7 C- Solutions 2 5 and 6 are bases solution 3 is an acid solution 1 is a salt and solution 4 can not be classified D- Solution 3 is a base solutions 2 5 and 6 are acids and solutions 1 and 4 are salts 8 Identify the following pH as either acid base or salt pH 6 pH 8 pH 14 pH 2 pH 11 pH 7 9 A 100 L buffer solution is 0250 M in HF and 0250 M in LiF Calculate the pH of the solution after the addition of 0150 moles of solid LiOH Assume no volume change upon the addition of base You c • The definition of pH is pH is equal to the negative log logarithm of the ion concentration of a solution • The logarithm of a number is the power to which 10 must be raised to This pH change occurs even with an inert salt such as NaNO 3 or MgCl 2 which we will examine in this example appearing here Example 2 Calculation of the true pH of a MgCl 2 solution Electrolytes are aqueous solutions that conduct electricity Acids bases and salts ionic compounds are electrolytes Nonelectrolytes are aqueous solutions that do not conduct electricity The solutes used to form nonelectrolytes are covalently bonded Classify the following as conductors or nonconductors by writing C or N next to each Name
Ph of salt solutions worksheet answers













0 Response to "35 Ph Of Salt Solutions Worksheet Answers"
Post a Comment