34 Understanding The Rutherford Model Worksheet Answers
Students will develop a model that illustrates the arrangement of electrons using the Bohr Model and the Lewis Dot Diagram. Bohr Model Notes. Used to show arrangement of electrons. Electrons are placed on the _____ shell first. Bohr Model Worksheet. Use the description sheet and the periodic table to help you complete the following Bohr... About This Quiz & Worksheet. With this quiz and accompanying worksheet, you will get a more detailed understanding of Rutherford's Gold Foil Experiment and the structure of the atom.
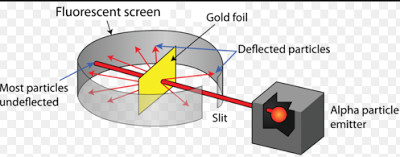
Understanding the rutherford model worksheet answers. Directions below is a representation of rutherfords gold foil experiment. In this rutherford model worksheet students read about rutherfords experiment with gold foil and alpha particles which led to the discovery of subatomic particles.
Understanding the rutherford model worksheet answers
This Understanding the Rutherford Model Worksheet is suitable for 9th - 12th Grade. In this Rutherford model worksheet, students read about Rutherford's experiment with gold foil and alpha particles which led to the discovery of subatomic particles. Students answer six analysis questions about the experiment and subatomic particles. batomic Particles Complete the chart using the given information and the Periodic Table. All entries relate to atoms. Element name aluminum carbon STUDENT JOURNAL - Week 8- Understanding the Atom. Once you have finished your Guided Practice work, move on to the Independent Worksheet at the end of this packet • CCM - Chapter 4; Lesson 1... • Rutherford's model did not answer these key questions:
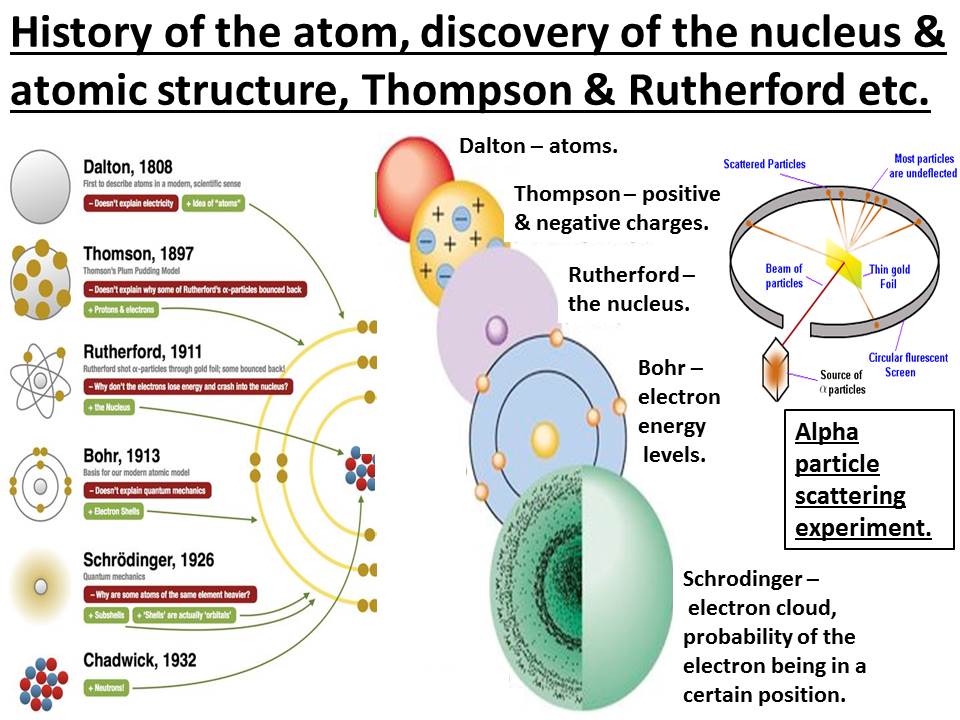
Understanding the rutherford model worksheet answers. Circle the correct answer. 15. In Thomson’s “plum-pudding” model, electrons are NOT. c. collected together in the center of the atom. Rutherford Opens an Atomic. 16. Before his experiment, Rutherford expected the particles to deflect to the sides of the gold foil. False. 17. Atomic Model of Matter Worksheet and key 5. Atom Notes 6. Complete Model of Atom Graphic Organizer and Key 7. Vocabulary Review and Key 8. Periodic Table 9. Understanding the Atom - Finding Numbers of Protons, Neutrons, Electrons and Key 10. Drawing Bohr Models of Atoms 1 - 20 and Key. Rutherford Model - 1908 Bohr Model - 1913 Wave Model... Rutherford had transmuted one element into two others - he had become an alchemist and radium, with its powerful radioactivity, was the philosopher's stone. The press hailed Rutherford as the first alchemist but in fact that was the least of it. What alchemy had shown him was the inside, not just of the atom, but of the strange object at 14. Outline the contributions of Dalton, Thomson, Rutherford and Bohr to the present understanding of atomic structure. For each, describe their experiment and model of the atom. Dalton: revived the atomic theory and got science back on track stated that all matter is made of atoms
About This Quiz & Worksheet. Some of the questions will test you on the format of the Rutherford model of the atom. It will ask you about the location of different particles in the model. Lesson Plan For Rutherford Scattering Building A Model Of The Atom Section 51 light and quantized energy. 51 models of the atom worksheet answers. 512 identify the new proposal in the bohr model of the atom. That quest continues in this chapter as scientists pursued an understanding of how electrons were arranged within atoms. Preschool Worksheets History Of The Atomet Answer Key Preschoolets. Toxic Science. Rontavstudio Drawing Atoms Worksheet Answer Key Inspirational. Understanding The Rutherford Model Section 7 Atomic Theory. Hiroshima Atomic Bomb Aug 06 1945 Worksheet Free Esl Printable. Section 4 1 Studying Atoms. (a) Give a concise explanation that shows clear understanding of the development of the model of the atom from Dalton to Thomson to Rutherford. The Dalton model proposed that matter was made of indivisible atoms / smallest building block of matter (The Dalton model had no electrons or protons).
Worksheet Background Ernest Rutherford was born in 1871 in New Zealand. In 1894, Rutherford traveled to England to work as a research student under J.J. Thomson. In 1898, while at Cambridge University, Rutherford discovered the existence of alpha and beta rays in uranium. In 1908, Rutherford's basic model by proposing that electrons had set energy levels (Fig. 7). This is the model of the atom most commonly portrayed in textbooks: a nucleus orbited by electrons at different levels. It helped solve the problem of the collapsing atom and earned Bohr a Nobel Prize. Just as Bohr built on Rutherford's model, many other Understanding Rutherford Model Worksheet Answers. January 10th, 2013 12:03:05 PM. Senior 1 - Education and Literacy. u2022 a student-generated test or series of questions and answers. Bohr-Rutherford Diagrams Worksheet... molecular model kits to further their understanding of ... [Filename: cluster2.pdf] - Read File Online - Report Abuse. • Students will have the opportunity to apply their knowledge and understanding through a level of response question. Associated materials: • The Superheroes of the Atomic Model Teacher Pack • The Superheroes of the Atomic Model Learner Worksheet - Foundation and Higher (Higher includes Gieger and Marsden) qualifications being deve
ernest rutherford performed an experiment 1911 that helped him develop the. solar model of the atom. he probed the inside of the atom using small, positively charged particles called alpha particles. based on his observation, he suggested that the atom is. mostly empty space with a small, positively charged center and negatively charged.
This Understanding the Rutherford Model Worksheet is suitable for 9th - 12th Grade. In this Rutherford model worksheet, students read about Rutherford's experiment with gold foil and alpha particles which led to the discovery of subatomic particles. Students answer six analysis questions about the experiment and subatomic particles.
NOTE: You may want have students answer question 1 on The Smallest Matter Worksheet first. 5. Conclude by asking students how the Rutherford-Bohr Theory of Atomic Structure Model helps us understand atomic structure today. The theory provides us with the understanding that an atom has a dense, positively-charged nucleus and that
Understanding the Rutherford Model Ernest Rutherford performed an experiment in 1911 that helped him develop the solar system model of the atom. He probed the inside of the atom using small, positively charged particles called alpha particles. Based on his observations, he suggested that the atom is
STUDENT JOURNAL - Week 8- Understanding the Atom. Once you have finished your Guided Practice work, move on to the Independent Worksheet at the end of this packet • CCM - Chapter 4; Lesson 1... • Rutherford's model did not answer these key questions:
batomic Particles Complete the chart using the given information and the Periodic Table. All entries relate to atoms. Element name aluminum carbon
Understanding the Rutherford Model; Inside an Atom; Understanding the Bohr Model; The Bohr Model vs the Wave Mechanical Model; Drawing Atomic Diagrams. Number of Neutrons; Analyzing the Bohr Atom; Electron Dot Diagrams, Etc. Average Atomic Mass; Where Are the Electrons.
Chemistry: Form WS2.2.1A Name _____ ATOMS Date _____ Period _____ Understanding the Rutherford Model Ernest Rutherford performed an experiment in 1911 that helped him develop the solar system model of the atom. He probed the inside of the atom using small, positively charged particles called alpha particles. Based on his observations, he suggested that the atom is mostly empty space with a.
Drawing Dohr model diagrams 1. Refer to the Bohr model chart on page 32 to help you complete the following table. Some answers are provided for you. (Hint: Remember that the maximum number of electrons in the first three shells is 2, 8, and 8.) Number of electrons 10 10 10 14/ 18 18 Number of electron shells Atom/ion neon atom fluorine atom
SNC1P BOHR DIAGRAM WORKSHEET NAME: Use the information in your 'Atomic Number, Mass Number Worksheet' to help you draw complete Bohr Diagrams for the following twenty elements. Some have been done for you. electron 2P number of protons 2P 1P orbital shells 2N number of neutrons 2N.
Section 7: Atomic Theory [UNDERSTANDING THE RUTHERFORD MODEL] Graham Mueller Hour 8 Ernest Rutherford performed an experiment in 1911 that helped him develop the solar system model of the atom. He probed the inside of the atom using small, positively charged particles called alpha particles. Based on his observations, he suggested that the atom is mostly empty space with a small, positively.
Understanding the Rutherford Model For Students 9th - 12th. For this Rutherford model worksheet, students read about Rutherford's experiment with gold foil and alpha particles which led to the discovery of subatomic particles.. In this history of the atom worksheet, students answer questions about Thomson's cathode ray apparatus, Rutherford...
Explain the History of the Atomic Model. In this worksheet, students will learn about how our understanding of the structure of the atom has changed, and some of the evidence that caused these changes. Key stage: KS 4. GCSE Boards: AQA, AQA Trilogy, OCR 21st Century, OCR Gateway, Pearson Edexcel, Eduqas, AQA Synergy,
Lesson Plan For Rutherford Scattering Building A Model Of The Atom Section 51 light and quantized energy. 51 models of the atom worksheet answers. 512 identify. don't understand. Understanding the Atom Discovering Parts of an Atom LESSON 1 CHAPTER 7 Reading Essentials Understanding the Atom 115. Introduction.
Bohr model and lewis dot diagram worksheet answers periodic table worksheets doc new blank bohr model worksheet fill in for first 20 elements and lewis dot diagram worksheet answers collection of structure with diagrams bohr and lewis models as well some naming review we marked went along the key is below if you didn t finish it homework.
2. Complete the table in Model 1 by counting the protons and neutrons in each atomic diagram. Divide the work evenly among group members. See Model l. 3. Find the three elements shown in Model 1 on your periodic table. a. What whole number shown in Model 1 for each element is also found in the periodic table for that element? Hydrogen — I.



























0 Response to "34 Understanding The Rutherford Model Worksheet Answers"
Post a Comment