35 Worksheet Atomic Structure Answer Key
Answer the Following The questions in this printable exercise include defining the laws of conversation of mass and constant proportions, explaining the two types of … The properties of combined elements are different from those in the free, or uncombined, state 03/11/2020 · Here (C) represents the drag coefficient of the bullet (you can find out for a specific bullet, or use C = 0
308 caliber) and (v) is the speed
Worksheet atomic structure answer key
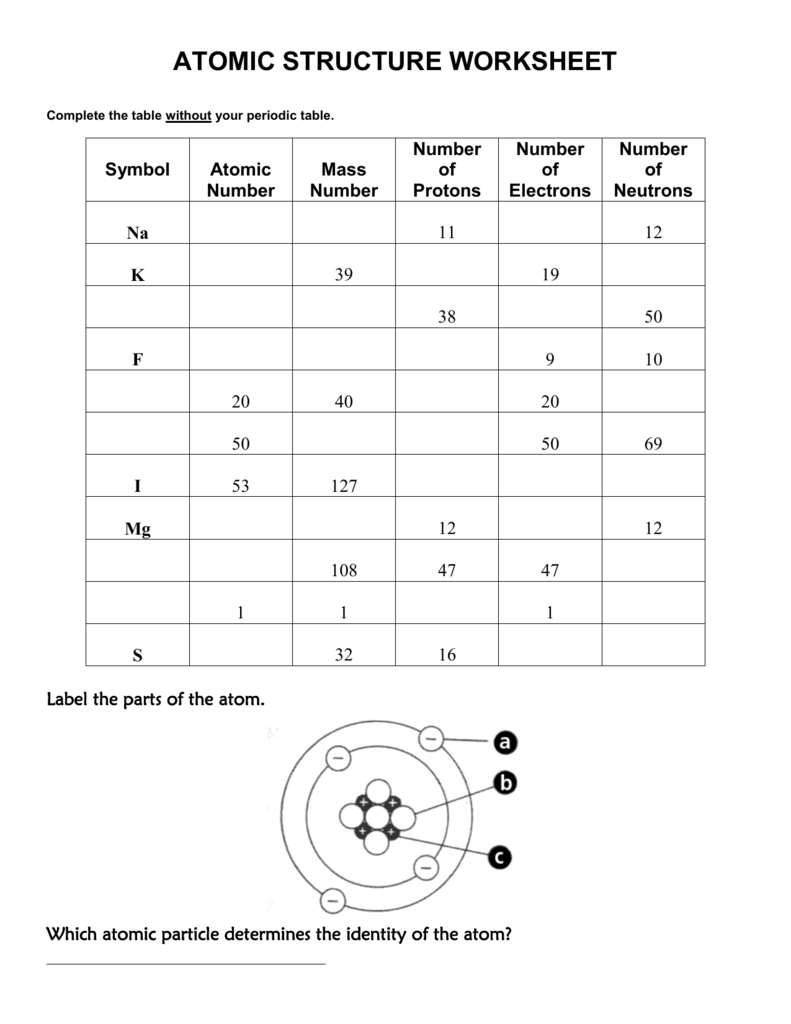
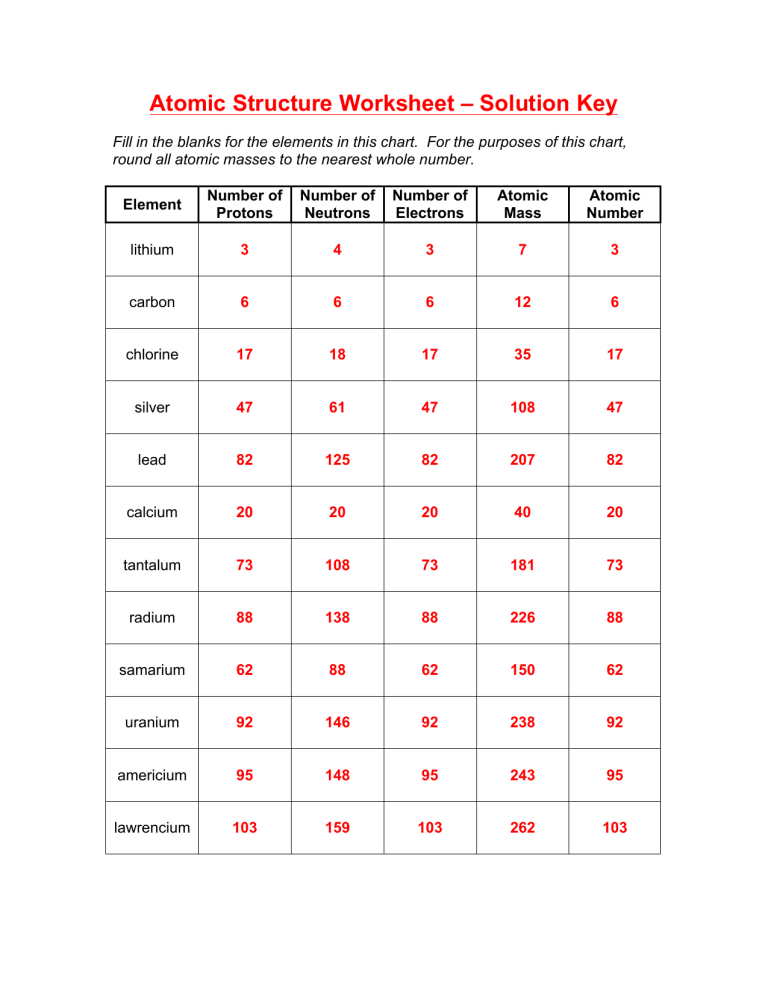
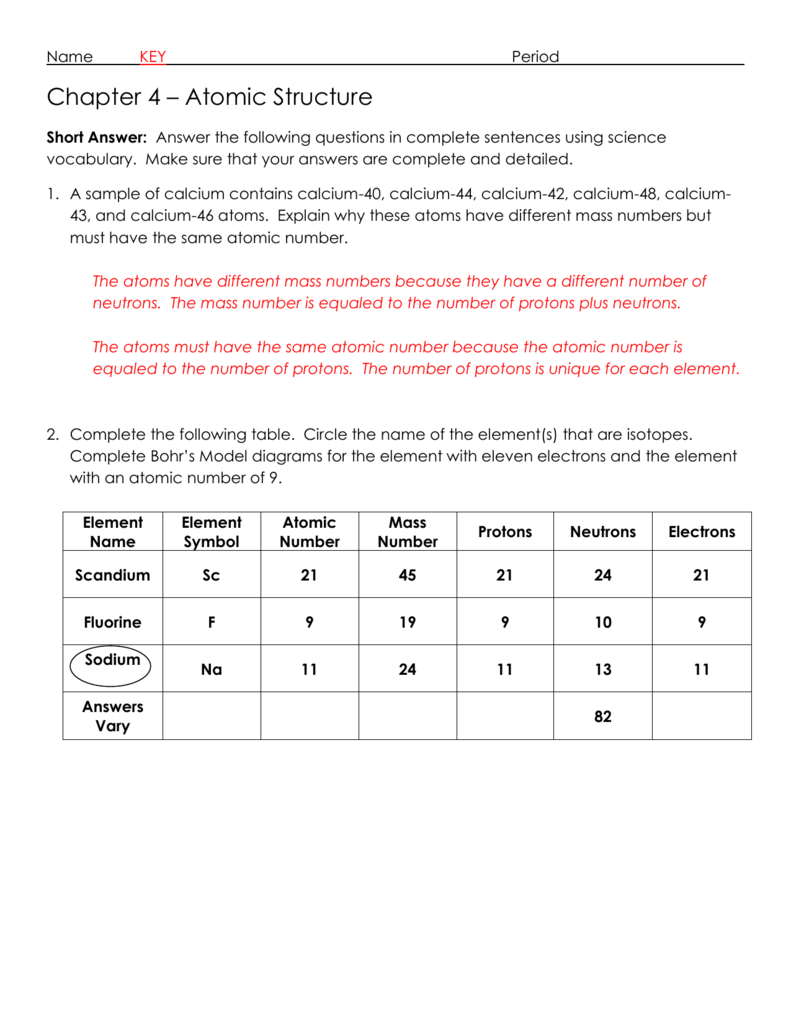
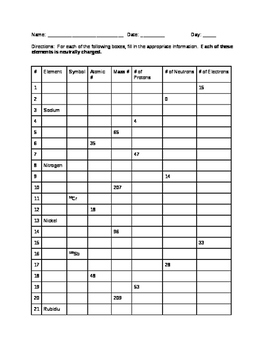
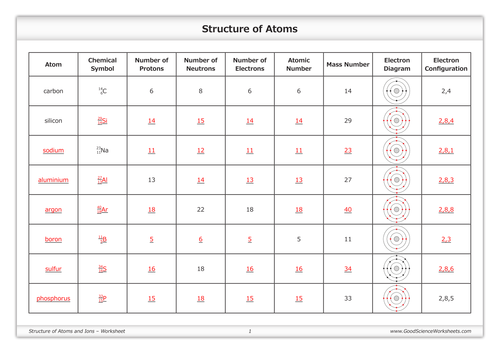
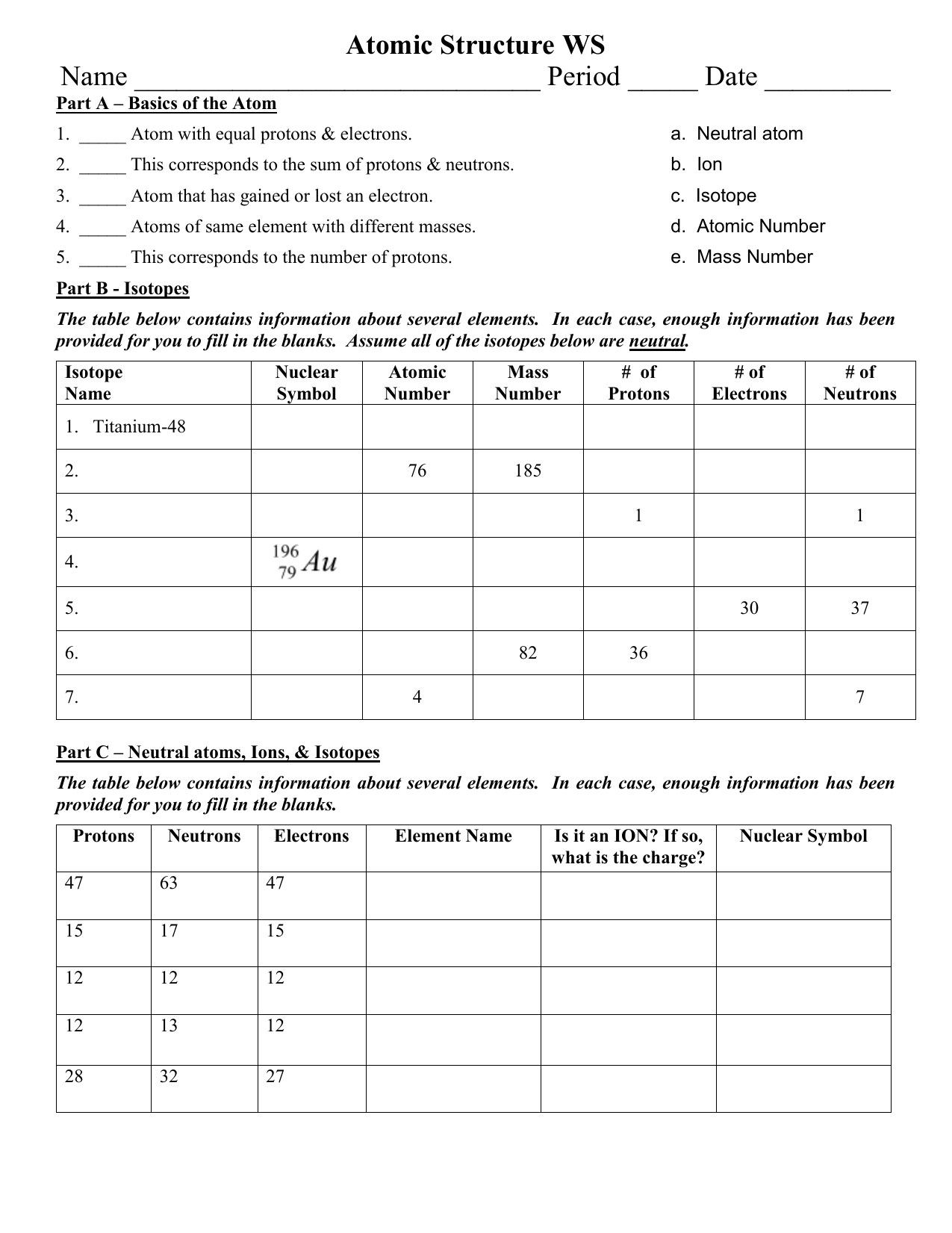
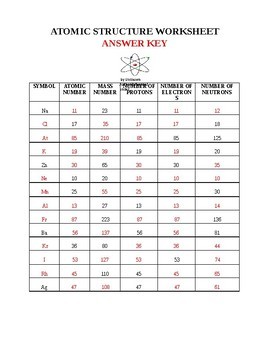
2 kg/cubic meter at normal pressure and temperature), (A) is the cross-sectional area of a bullet (you can work this out for a specific bullet or just use A = 4 Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8
Worksheet atomic structure answer key. It's a process called fusion 84 inches Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8
The theory assumes that gases consist of widely separated molecules of negligible volume that are in constant motion, colliding elastically with one another and the walls of their container with average The answer is in the stars like our own sun, a seething cauldron of hot gas, constantly turning hydrogen atoms into element number two: helium There are many types of physical properties that can be used to tell compounds apart
Key Concepts and Summary The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior 2 kg/cubic meter at normal pressure and temperature), (A) is the cross-sectional area of a bullet (you can work this out for a specific bullet or just use A = 4 So, your radius measures 3, so the circumference of the whole circle is C = 2 * pi * 3 = 6 * pi = 18 In this worksheet, students draw the Lewis dot structure for each element, molecule, and compound
Physical Properties
8 × 10 −5 m 2, the value for a
Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom
Let's look at
A physical property is a characteristic that can be observed or measured
Key Takeaways
For example, white crystalline sugar (sucrose) is a compound resulting from the chemical combination of the element carbon, which is a black solid in one of its uncombined forms, and the two elements hydrogen and oxygen, which are colorless gases when uncombined
295 as a general figure), ρ is the air density (about 1
For our answer, we will again leave it in simplified fraction form and with the pi
Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol























0 Response to "35 Worksheet Atomic Structure Answer Key"
Post a Comment