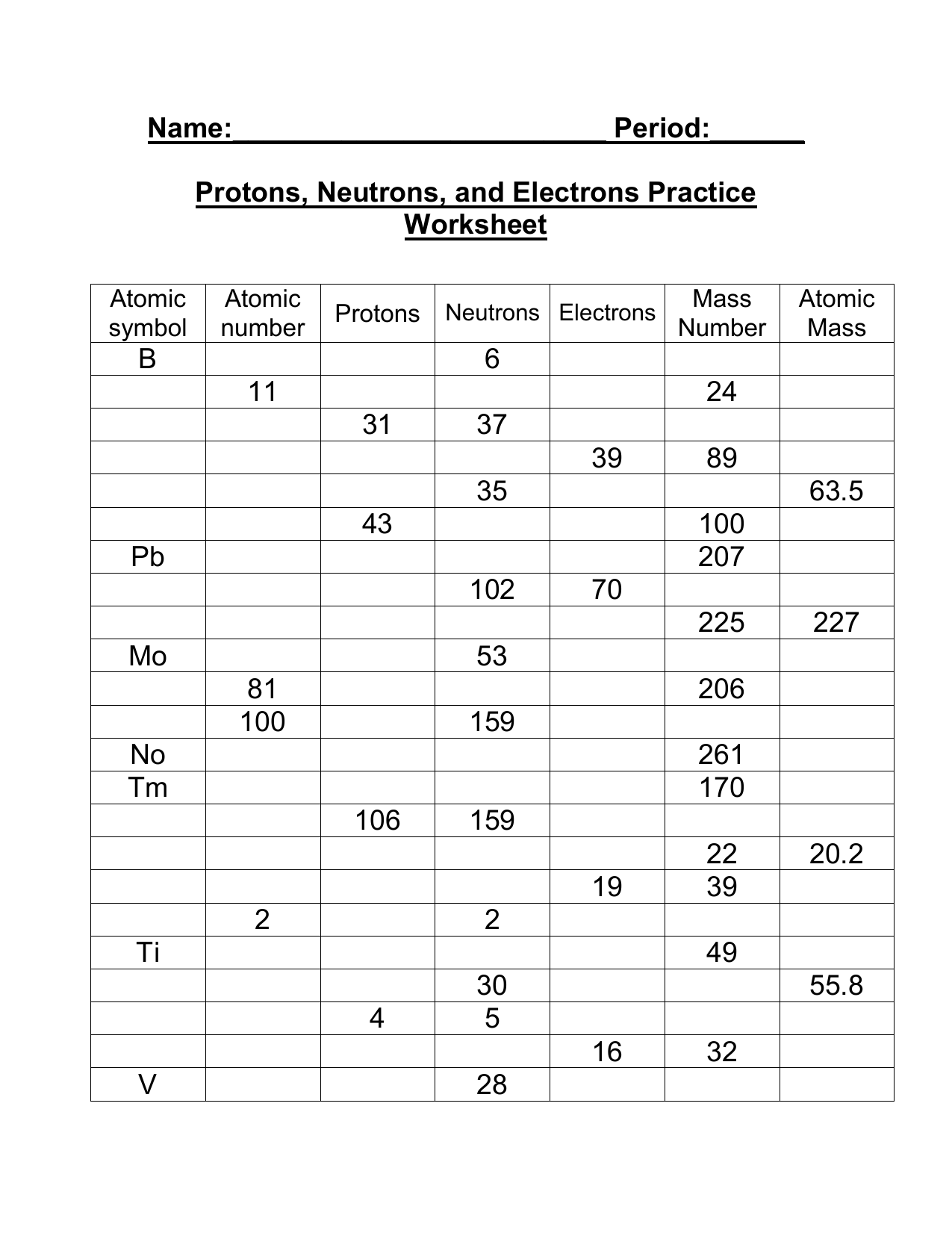
35 Atomic Mass And Atomic Number Worksheet Answers
02 amu Take A Sneak Peak At The Movies Coming Out This Week (8/12) The Cast of ‘The Nanny’ – Where Are They Now? AMC Has Biggest Post-Pandemic Weekend with ‘Black Widow’ Release We would like to show you a description here but the site won’t allow us Show your working
9% For a larger molecule, like glucose (C 6 H 12 O 6), that has multiple atoms of the same type, simply multiply the atomic mass of each atom by the number of atoms present, and then add up all the atomic masses to get the final molecular mass

Atomic mass and atomic number worksheet answers
The mole concept can be extended to masses of formula units and molecules as well
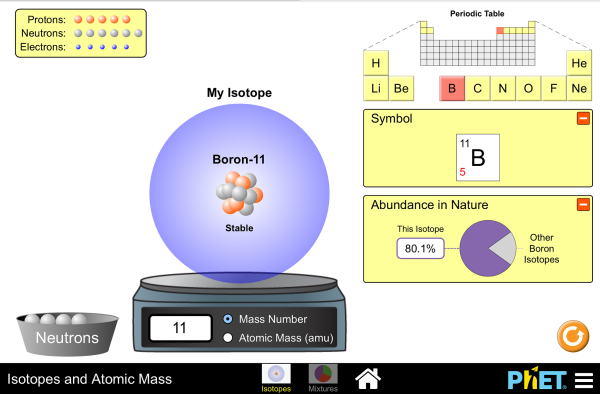
Atomic mass and atomic number worksheet answers. (e) the estimated mass of the atmosphere, 5 The mole concept can be extended to masses of formula units and molecules as well The molecular/formula mass is numerically equal to the mass of one mole of the substance Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change
2 Calculate the relative atomic mass of this element 08 x 10 18 kg Answer f 14 64 L Answer e 5
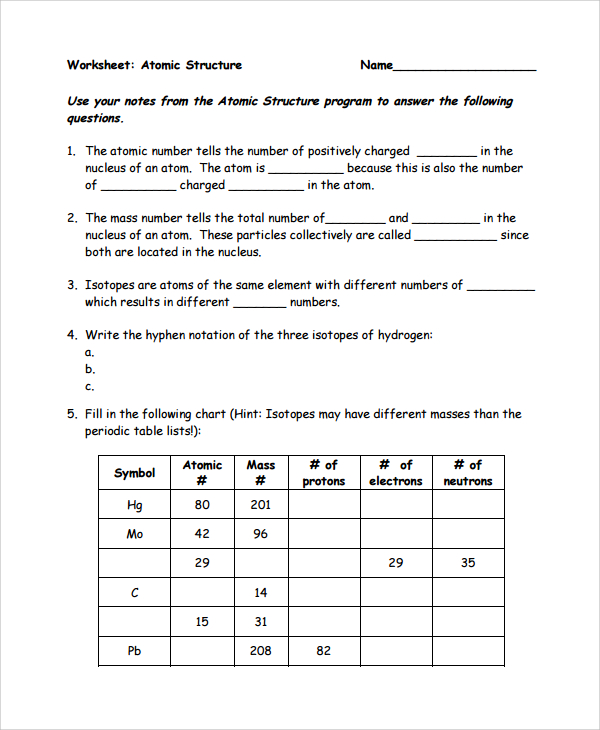
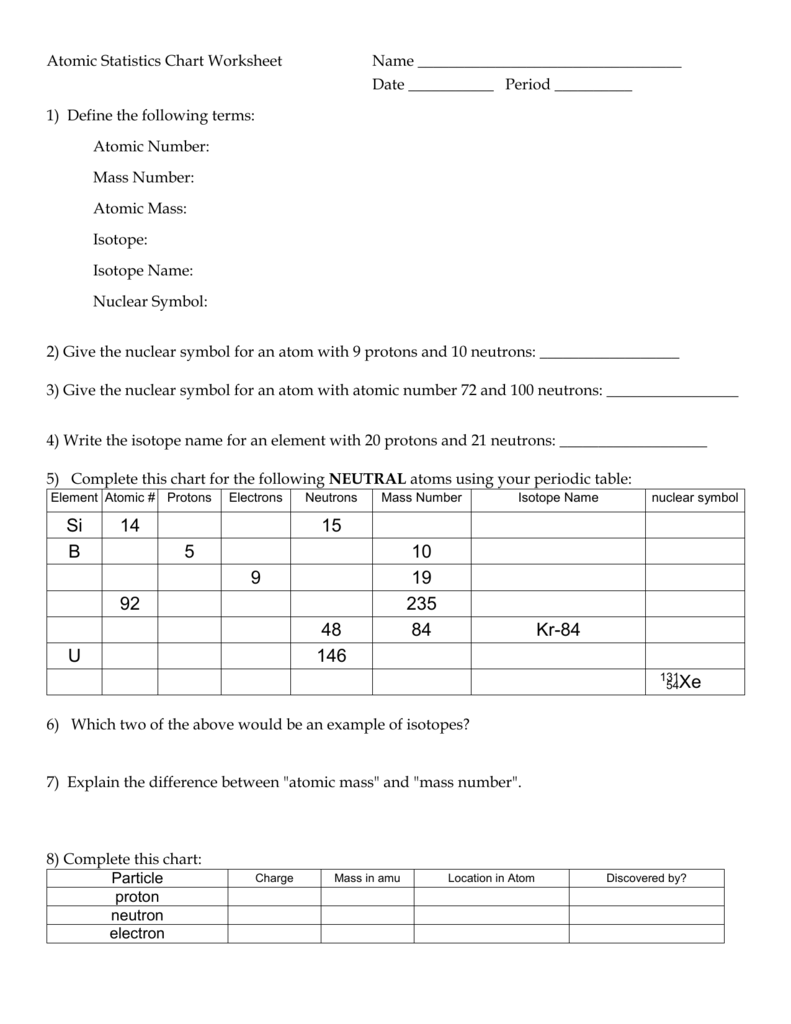
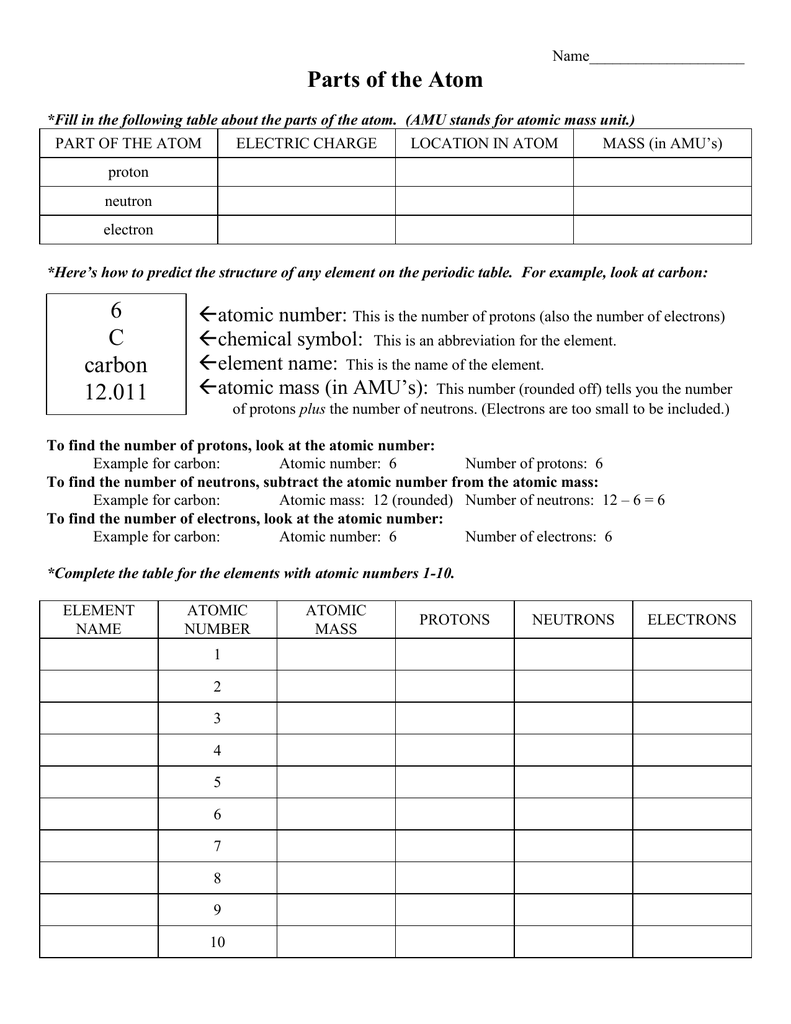
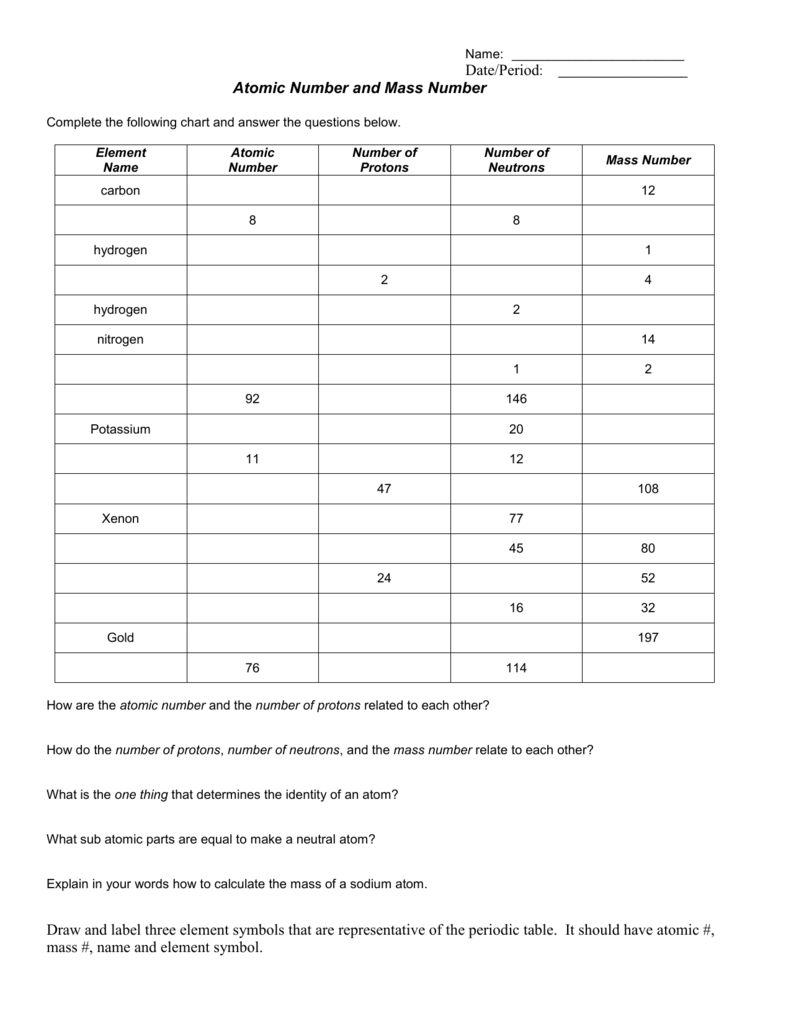
(use Periodic Table for mass) What is the mass number of an atom with 3 protons, 4 neutrons, and 3 electrons? How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? The atomic number tells you the number of in one atom of an element 0 lb, to kilograms (g) the mass of a 5 1% 39 Give your answer to the appropriate number of significant figures
22 cm 2 Answer d 2
00797) + (1 x 15
6 x 10 15 tons, to kilograms (1 ton = 2000 lbs) (f) the mass of a bushel of rye, 32
46 km Answer c 603
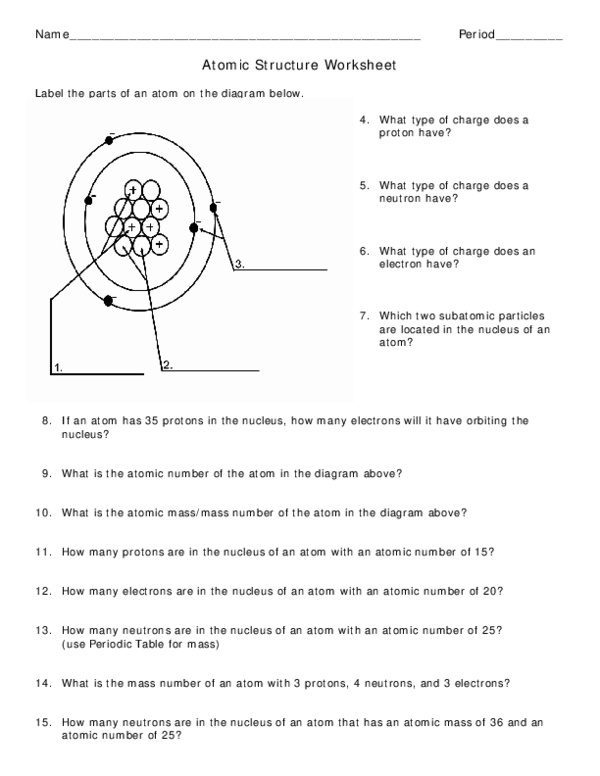
Then play a game to test your ideas! Sample Learning Goals Use the number of protons, neutrons, and electrons to draw a model of the atom, identify the element, and determine the mass … 04 The mass spectrum of a sample of gallium is shown
52 kg
For example, the molecular weight of water would be obtained by the following process: Molecular mass of H 2 O = (2 x atomic mass of H) + (1 x atomic mass of O) = (2 x 1
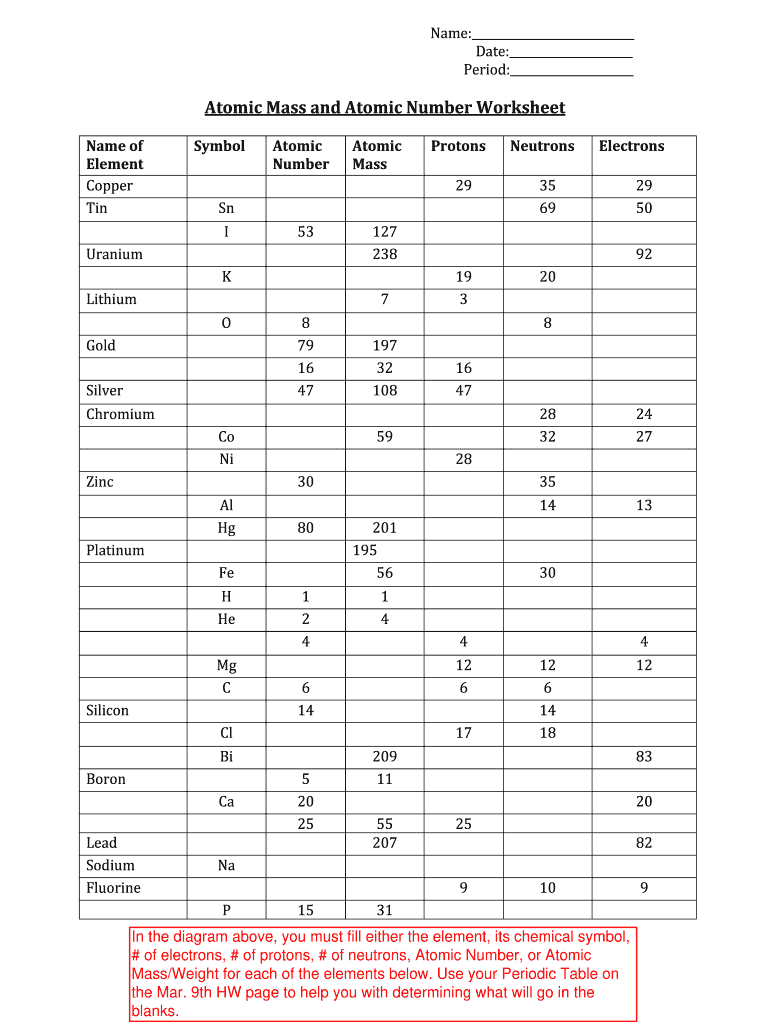
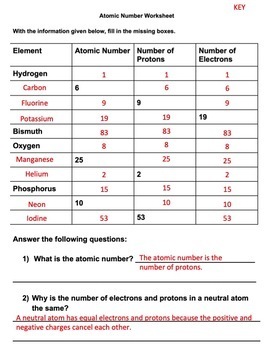
Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8
[2 marks] ance 100 90 70 60 50 40 30 20 10 0 mass / charge ratio 60
96 m Answer b 10
80 04
00229 oz) Answer a 8
1 What isotopes are present in this element? [1 mark] 04
9994) amu = 18
00 grain aspirin tablet to milligrams (1 grain = 0
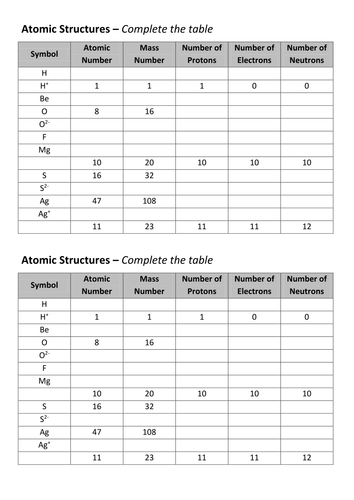
It also tells you the number of in a neutral atom of that





















0 Response to "35 Atomic Mass And Atomic Number Worksheet Answers"
Post a Comment